Session Information
Date: Saturday, November 16, 2024
Title: Abstracts: RA – Diagnosis, Manifestations, & Outcomes I: Breathe: RA-ILD
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Neutrophil activation is seen in rheumatoid arthritis (RA), but its involvement in RA interstitial lung disease (RA-ILD) is not clear. Levels of N-formyl methionine (fMet), a potent neutrophil activating factor signaling through formyl peptide receptor 1 (FPR1), have been reported to be elevated in RA. The aim of the current study is to assess the role of fMet in neutrophil-mediated inflammation in the RA-ILD pathogenesis.
Methods: Levels of fMet and calprotectin were analyzed by ELISA in plasma from healthy controls (n=50) and patients with RA-ILD (n=50), RA-no-ILD (n=49), chronic obstructive lung disease (COPD, n=50), idiopathic pulmonary fibrosis (IPF, n=50), and systemic sclerosis-associated ILD (SSc-ILD, n=20). Sputum samples from healthy controls (n=50), RA-ILD (n=19) and RA-no-ILD (n=25) were also analyzed. Capacity of plasma to activate control neutrophils in presence or absence of anti-FPR1 antibody was measured by flow cytometry analysis assessing up-regulation of CD11b and CD66b levels. Statistical analyses were done using Mann-Whitney U test and Spearman’s correlation.
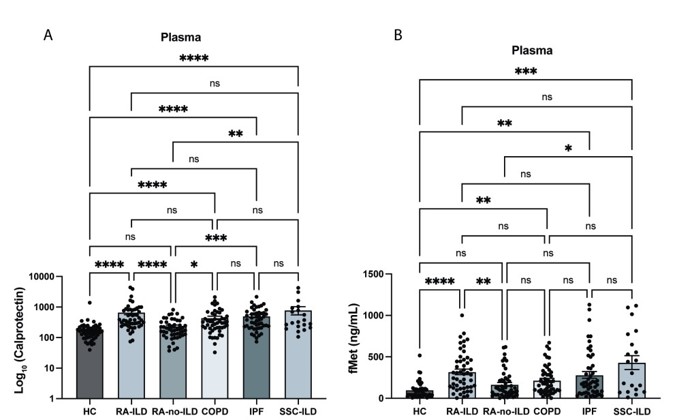
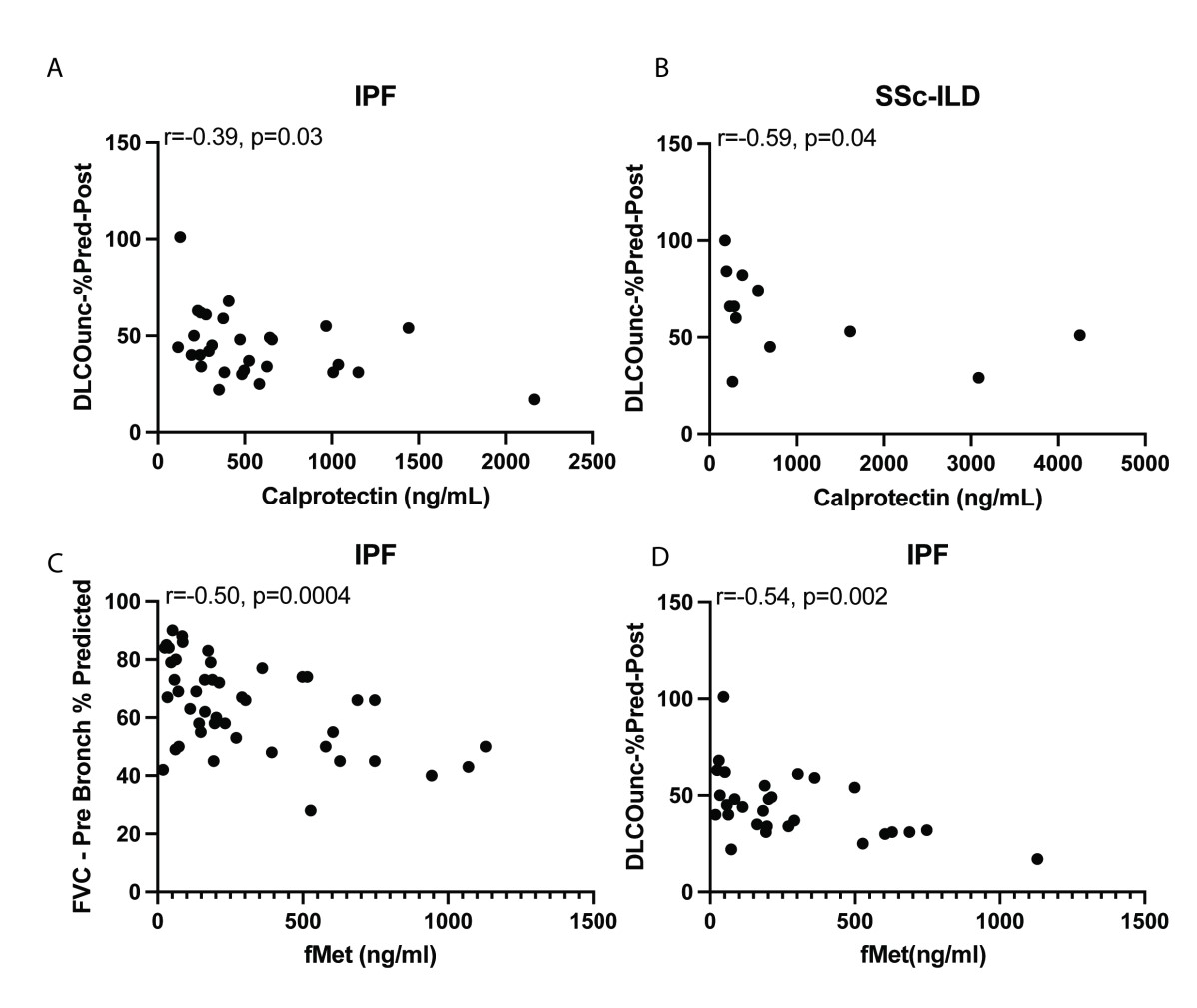
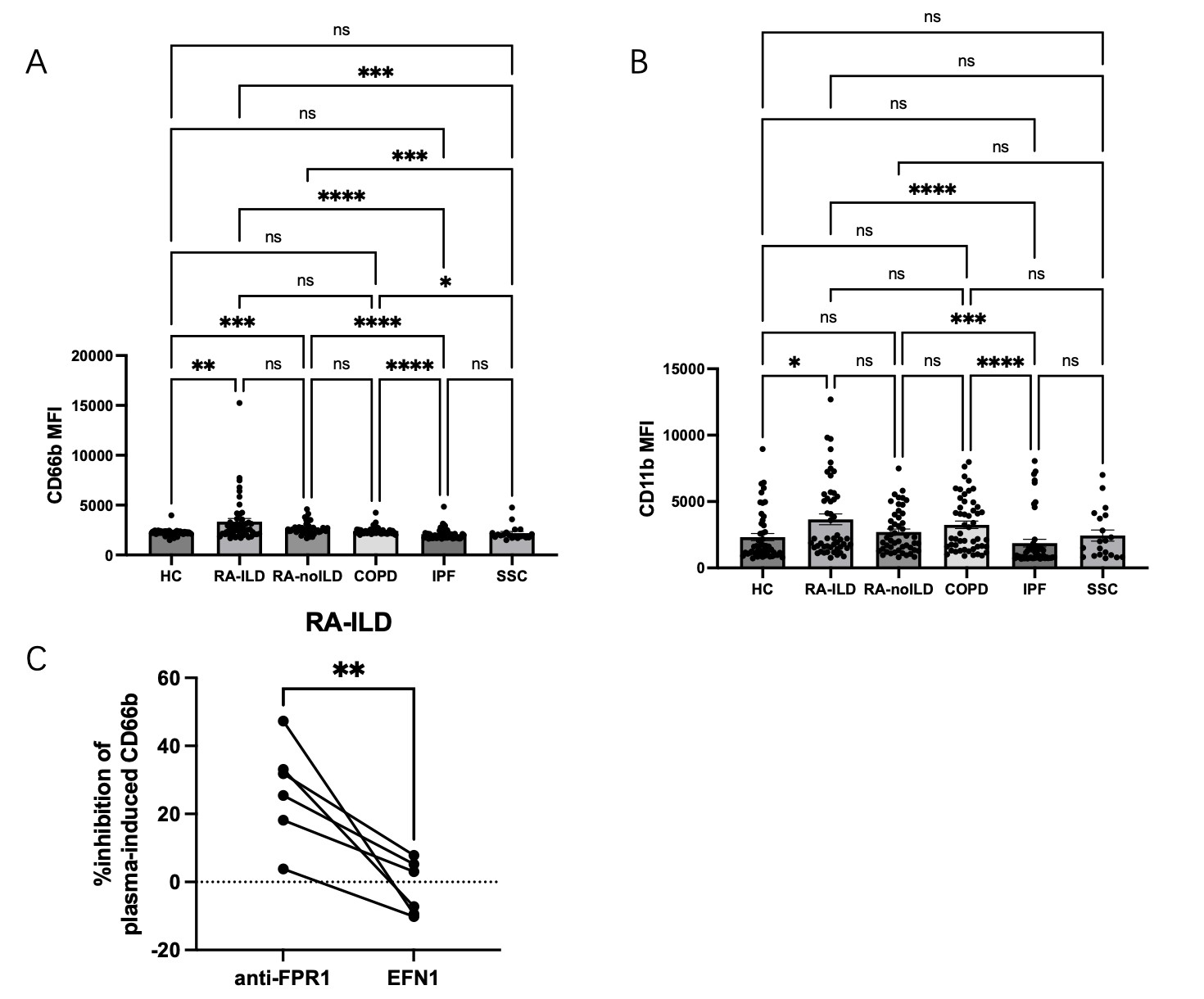
Results: Plasma levels of calprotectin were increased in RA-ILD, COPD, IPF, and SSc-ILD as compared to healthy controls (p< 0.0001 for all groups, Figure 1) as well as in RA-ILD vs RA-no-ILD (p< 0.0001). Levels of calprotectin were similarly elevated in sputum from RA-ILD vs healthy controls (p< 0.0001). In IPF and SSc-ILD, levels of calprotectin in plasma corresponded to lung function as determined by DLCO (r=-0.39, p=0.03; and r=-0.59, p=0.04, respectively, Figure 2). Levels of fMet were elevated in all lung disease groups, including in RA-ILD as compared to healthy controls (p< 0.0001). Of note, levels of calprotectin correlated with levels of fMet in RA-ILD (r=0.43, p=0.002). Similar findings were seen in the other disease groups. In addition, in IPF, levels of fMet in plasma corresponded to lung function as defined by FVC and DLCO (r=-0.50, p=0.0004; and r=-0.54, p=0.002, respectively, Figure 2). Finally, plasma from RA-ILD patients (but no other disease control), increased neutrophil activation in vitro as assessed by up-regulation of CD11b (p< 0.05) and CD66b (p< 0.01) levels, through FPR1-dependent mechanisms (Figure 3).
Conclusion: Neutrophil activation is prominent in blood and sputum of patients with lung involvement, with levels of calprotectin corresponding to lung capacity. Plasma samples from RA patients had increased fMet levels, as well as enhanced capacity to support neutrophil activation through FPR1-dependent mechanisms, consistent with a role for fMet/FPR1 signaling in RA-ILD pathogenesis. Further studies are warranted to determine the underlying mechanism(s) of neutrophil activation in ILD.
Levels of plasma A) calprotectin and B) fMet levels in healthy controls, RA-ILD, RA-no-ILD, COPD, IPF and SSC-ILD groups.
Correlation analysis between levels of calprotectin and DLCO in A) IPF, B) SSc-ILD. Correlation analysis between levels of fMet and C) FVC and D) DLCO in IPF.
Plasma-induced neutrophil activation in vitro assessed by A) CD11b and B) CD66b.
C) Percent inhibition of RA-ILD plasma induced neutrophil CD66b in the presence of anti-FPR1 or EFN1 (isotype of anti-FPR1).
To cite this abstract in AMA style:
Shi J, Wu Y, Wang T, Yu C, wang q, Tian X, Li M, Demoruelle K, Solomon J, Lood C. Neutrophil Activation as a Novel Marker of Lung Disease in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/neutrophil-activation-as-a-novel-marker-of-lung-disease-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/neutrophil-activation-as-a-novel-marker-of-lung-disease-in-rheumatoid-arthritis/