Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Conventional SLE diagnostic markers lack sensitivity and are biased towards severe disease, resulting in a subset of clinically ambiguous ANA-positive but specific autoantibody-negative patients. T Cell markers, including T Cell bound complement split product C4d (TC4d) and autoantibodies bound to T Cells (TIgG, & TIgM) demonstrate superior diagnostic accuracy in single-center studies. This multi-center study establishes the clinical validity and quantifies the added sensitivity in a cohort of SLE, other diseases and apparently healthy volunteers (AHV).
Methods: A cohort of 369 subjects inclusive of 91 SLE patients meeting 1997 ACR criteria, 159 with other autoimmune rheumatic diseases (OARD), 38 with other rheumatic diseases (ORD) and 81 AHV were enrolled. Whole blood was analyzed by flow cytometry for T Cell markers and cell-bound complement activation products (CB-CAPS) on erythrocytes and B cells (EC4d & BC4d). Conventional SLE markers (anti-Smith & anti-dsDNA) were measured by ELISA and C3/C4 were measured by immunoturbidimetry. Diagnostic cutoffs for T Cell markers were set at the 95th percentile of AHVs. Sensitivity and specificity for SLE vs. OARD, ORD and AHV were calculated. An overlap analysis using a multianalyte panel (MAP) algorithm integrating EC4d, BC4d and conventional markers was performed. ROC analysis was used to compare the discriminatory power of T Cell and conventional markers. A combined ROC curve for T Cell markers (T Cell marker algorithm) was created using logistic regression to predict SLE diagnosis and assess combined discriminatory power.
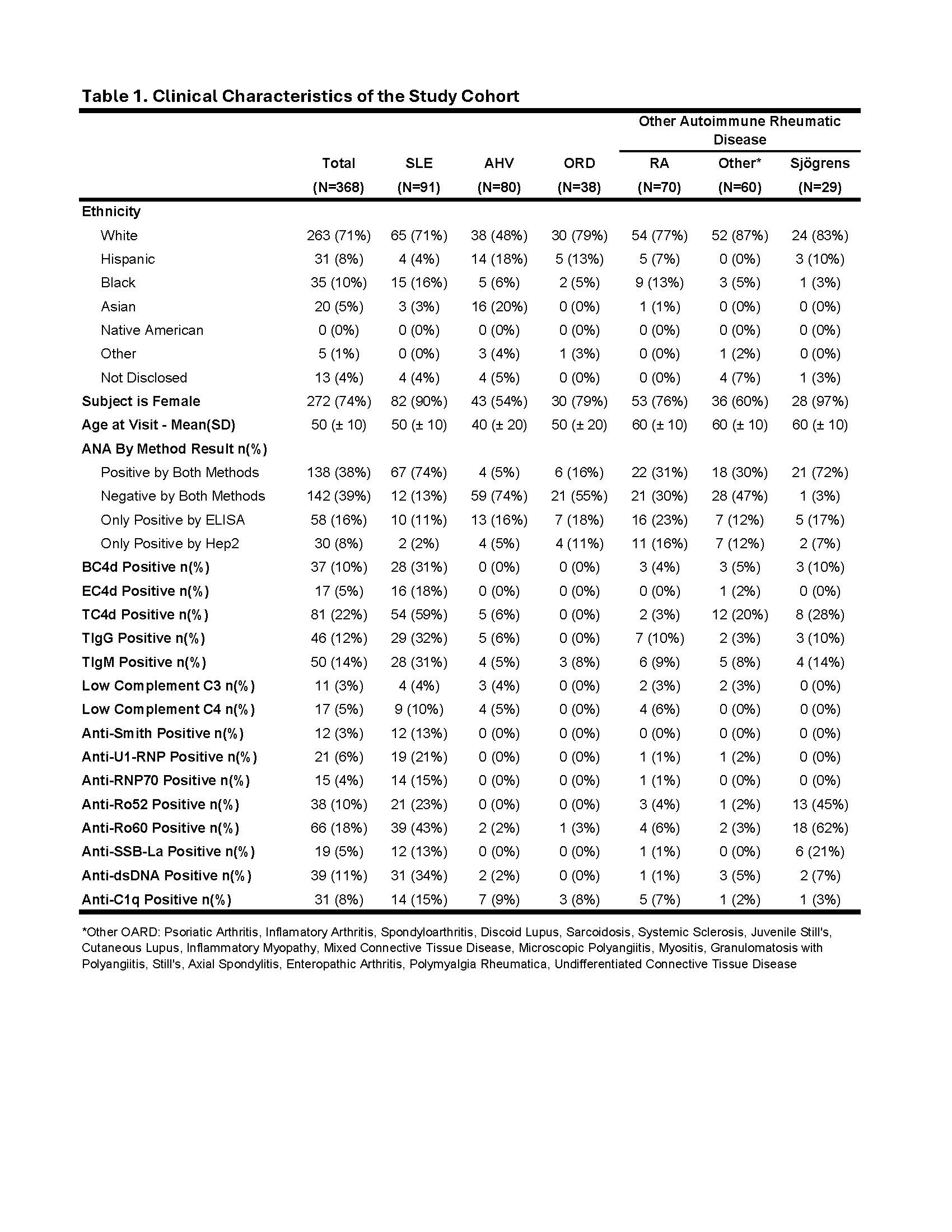
Results: The SLE cohort was majority white (71%), female (90%) and mean (SD) age of 50 (± 10) years old. AHVs were 48% white, 54% female and mean (SD) age of 40 (± 20) years old (Table 1). In diagnosing ANA positive (ANA+) SLE vs. OARD, area under the curve (AUC) values were TIgG (0.83), BC4d (0.81), T Cell marker algorithm (0.80), TC4d (0.78) and anti-dsDNA (0.73) (Fig. 1A). For diagnosing ANA+ SLE vs. AHV, ROC analysis comparing T Cell markers and CB-CAPs to conventional SLE markers yielded the following AUCs: BC4d (0.89), EC4d (0.84), T Cell marker algorithm (0.84), TC4d (0.82) and anti-dsDNA (0.81) (Fig. 1B). Similar analysis for ANA-negative SLE versus AHV and OARD showed T Cell markers outperformed conventional markers (Fig. 1C & 1D). With specificity for SLE vs. AHV at 95%, sensitivity was 59%, 32% and 31% for TC4d, TIgG and TIgM, respectively. Among the 91 SLE subjects, 69 (76%) tested positive for one or more of EC4d, BC4d, conventional SLE markers or T Cell markers (Fig. 2A). Of 69 subjects with one or more marker positive, 23% were positive solely for T Cell markers, compared to 2% for TIgG and 1% for TIgM (Fig. 2B). The combination of MAP, conventional markers and T Cell markers demonstrated 80% sensitivity for SLE, with 22% uniquely positive for T Cell markers, 4% MAP, and 2% by conventional markers (Fig. 2C).
Conclusion: Integrating T Cell markers with conventional SLE markers and CB-CAPs enhances the detection of SLE, providing a more comprehensive approach to diagnosis. Incorporating T Cell markers into diagnostic protocols could significantly improve the identification and timely treatment of SLE, particularly in clinically ambiguous cases.
To cite this abstract in AMA style:
Kyttaris V, Concoff A, Warsi T, Taghavi S, Kumar S, Park S, Patalinghug A, Schleif C, Partain B, Ahearn J, Wilson N, Liu C, Manzi S, O'Malley T. Multi-centered Clinical Validation of T Cell-bound C4d (TC4d) and T Cell Autoantibodies (TIgG and TIgM): Sensitive and Specific Biomarkers of SLE with Enhanced Accuracy Compared to Conventional SLE Tests [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/multi-centered-clinical-validation-of-t-cell-bound-c4d-tc4d-and-t-cell-autoantibodies-tigg-and-tigm-sensitive-and-specific-biomarkers-of-sle-with-enhanced-accuracy-compared-to-conventional-sle-te/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/multi-centered-clinical-validation-of-t-cell-bound-c4d-tc4d-and-t-cell-autoantibodies-tigg-and-tigm-sensitive-and-specific-biomarkers-of-sle-with-enhanced-accuracy-compared-to-conventional-sle-te/