Session Information
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: CD38 can function as a degrading enzyme of nicotinamide adenine dinucleotide (NAD), a critical metabolic intermediate serving as enzyme cofactor in redox reactions and as a co-substrate by many enzymes such as sirtuins (SIRTs). We have recently observed that expression of CD38 is increased in PBMCs of gout patients compared to health controls, and inhibition of CD38 attenuated monosodium urate (MSU) crystal-induced acute inflammatory responses in mice in vivo. In this study, we investigated the molecular mechanism mediating beneficial effect of inhibition of CD38 in macrophages in response to MSU crystals in vitro.
Methods: Bone marrow derived macrophages (BMDMs) prepared from CD38 knockout (KO) and wild type (WT) were treated with MSU crystals (0.2 mg/ml) in the presence or absence of apigenin (25 μM), a flavonoid that selectively inhibits CD38 for 6 and 24 hours. Expression of CD38 was examined at both mRNA and protein levels by qRT-PCR and Western blot analysis. The NADH/NAD Quantification Kit (BioVision) was used to measure the intracellular NAD, NADH and their ratio. Western blot analysis was carried out to examine expression of NAD-dependent SIRT3 and acetylated SOD2, expression of NLRP3 and cleaved caspase-1. ELISA was performed to determine the release of IL-1β from the conditioned media. RNA sequencing analysis was also carried out to profile differentially expressed genes.
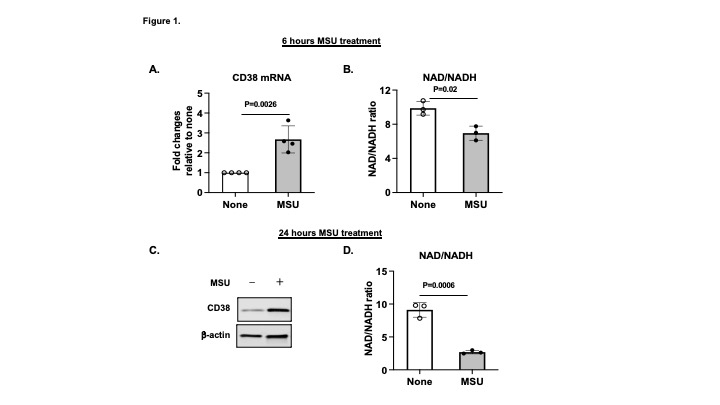
Results: The NAD/NADH ratio was significantly reduced in BMDMs treated with MSU crystals at both 6 and 24 hours, correlated with increased CD38 expression at both mRNA and protein levels, respectively. Apigenin inhibited CD38 expression, prevented a decrease in NAD/NADH ratio, prohibited a decrease in expression of SIRT3 and an increase in expression of acetylated SOD2, an antioxidant enzyme in the mitochondria, in BMDMs treated with MSU crystals. Apigenin also suppressed NLRP3 expression at both mRNA and protein levels, inhibited expression of cleaved casepase-1, and attenuated IL-1ß release in response to MSU crystals. RNA-seq analysis showed that 163 genes upregulated in WT+MSU BMDMs (log2FC≥1)were downregulated in both WT+MSU+apigenin and CD38KO BMDMs (log2FC≤1). KEGG Pathway enrichment analysis revealed several inflammatory pathways including cytokine-cytokine receptor interaction, chemokine, TNF, NF-κB, JAK-STAT, HIF-1α, TLR and MAPK signaling. Interestingly, a top differentially expressed gene six-transmembrane epithelial antigen of prostate 4 (Steap4) was upregulated in WT+MSU BMDMs (log2FC=8.14) but was downregulated in WT+MSU+apigenin BMDMs (log2FC= –7.18) and CD38KO+MSU BMDMs (log2FC= –4.43). Steap4 is a metalloreductase that has the ability to reduce both Fe(3+) to Fe(2+) and Cu(2+) to Cu(1+), using NAD(+) as acceptor. Increased Steap4 expression has been shown to lead to mitochondrial iron accumulation and enhanced reactive oxygen species production. Thus, downregulation of Steap4 expression induced by MSU crystals through inhibition of CD38 may help maintain mitochondria homeostasis.
Conclusion: Inhibition of CD38 can attenuate inflammatory response to MSU crystals in macrophages by prohibiting NAD decline and preventing mitochondrial dysfunction.
To cite this abstract in AMA style:
Qin H, Oliveira P, Yan T, Terkeltaub R, Liu Bryan R. Molecular Mechanism of Inhibition of CD38 in Attenuation of Monosodium Urate Crystal-induced Inflammatory Responses in Macrophages [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/molecular-mechanism-of-inhibition-of-cd38-in-attenuation-of-monosodium-urate-crystal-induced-inflammatory-responses-in-macrophages/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/molecular-mechanism-of-inhibition-of-cd38-in-attenuation-of-monosodium-urate-crystal-induced-inflammatory-responses-in-macrophages/