Session Information
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Upregulation of Type I interferons (IFN I), including IFNβ, is a hallmark of adult and juvenile dermatomyositis (JDM), but its role in pathogenesis is not clearly understood. Since IFN I activates the Janus kinase (JAK)-signal transducer and transcription (STAT) pathway, JAK inhibitors, including baricitinib (JAK 1/2 inhibitor) and tofacitinib (JAK 1/2/3 inhibitor), have therapeutic potential for JDM. Lack of adequate clinical trials, difficulty in obtaining routine JDM muscle biopsies, and absence of a disease model hinder understanding pathologic triggers and delay development of needed therapies. Using an in vitro 3D biomimetic construct derived from healthy pediatric muscle (termed “myobundles”), preliminary work demonstrated IFNβ-associated decrease in tetanus contractile force, slowed twitch kinetics, and paradoxically decreased fatigue.
- Aim: Determine functional effects of tofacitinib and baricitinib on myobundles exposed concurrently to IFNβ.
- Hypothesis: JAK inhibitors normalize IFNβ-associated decrease in tetanus contractile force but do not reverse changes in twitch kinetics or fatigue.
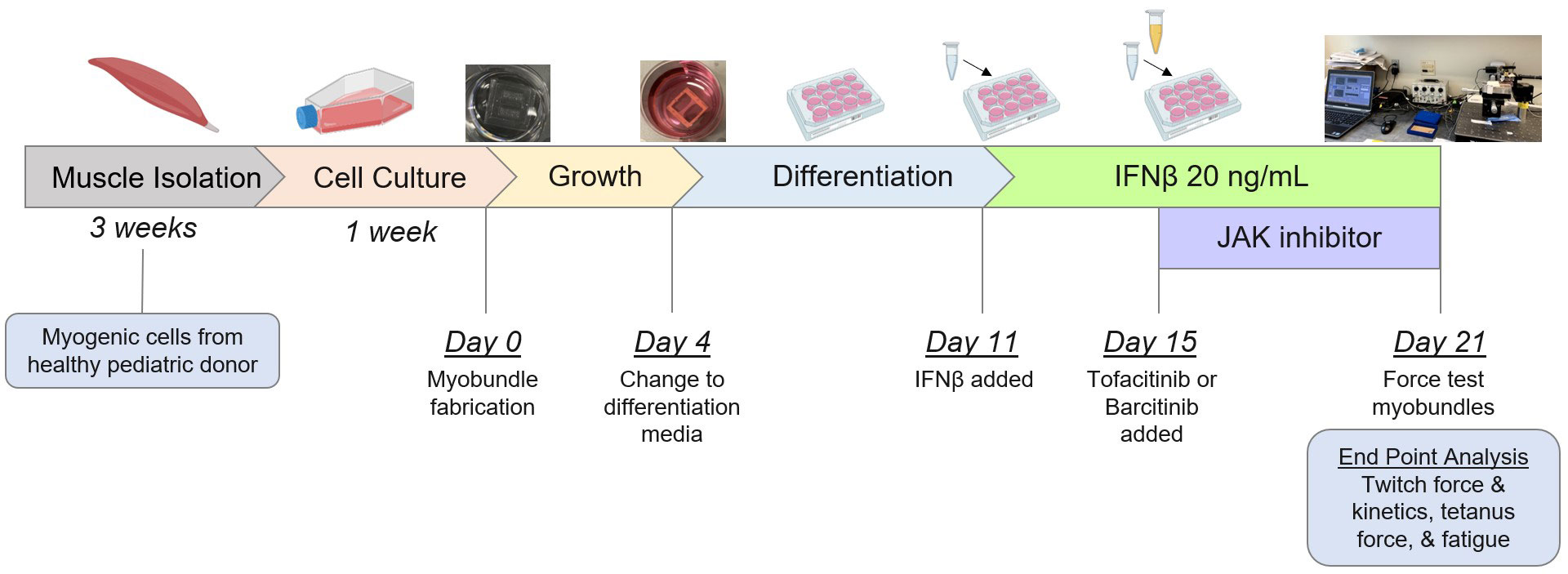
Methods: As seen in Figure 1, myogenic cells isolated from 2 healthy pediatric donors were cultured and used to create donor-specific myobundles based on established protocols (Madden, 2015). After growth and differentiation, myobundles were exposed to 0 (control condition) or 20 ng/mL IFNβ for a total of 10 days. Tofacitinib 1 uM (suspended in H2O) and baricitinib 500 nM (suspended in DMSO 0.01%) were introduced to culture media during the last 6 days of IFNβ exposure. Control myobundles were treated with dimethyl sulfoxide (DMSO) 0.01% at this same timepoint. On day 21 after myobundle creation, contractile force after twitch (1 Hz for 10 ms), tetanus (20 Hz for 1 s), and fatigue (20 Hz for 30 s) electrical stimulation was measured. To assess kinetics, time to reach maximum force and half-relax were measured after twitch stimulation. Fatigue percentage was determined by reduction in force after fatigue stimulation. Data was analyzed using one-way ANOVA with multiple post hoc comparisons.
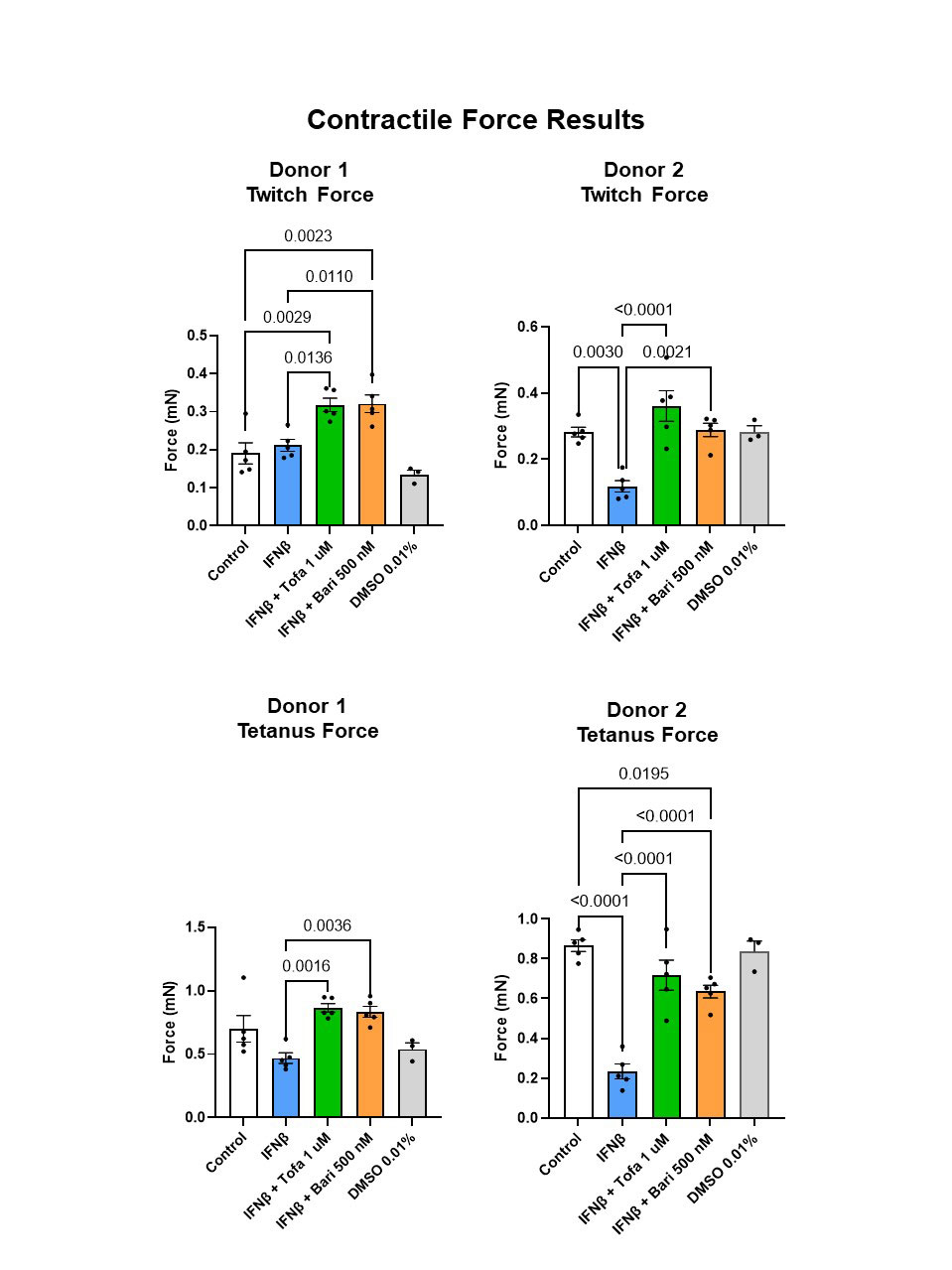
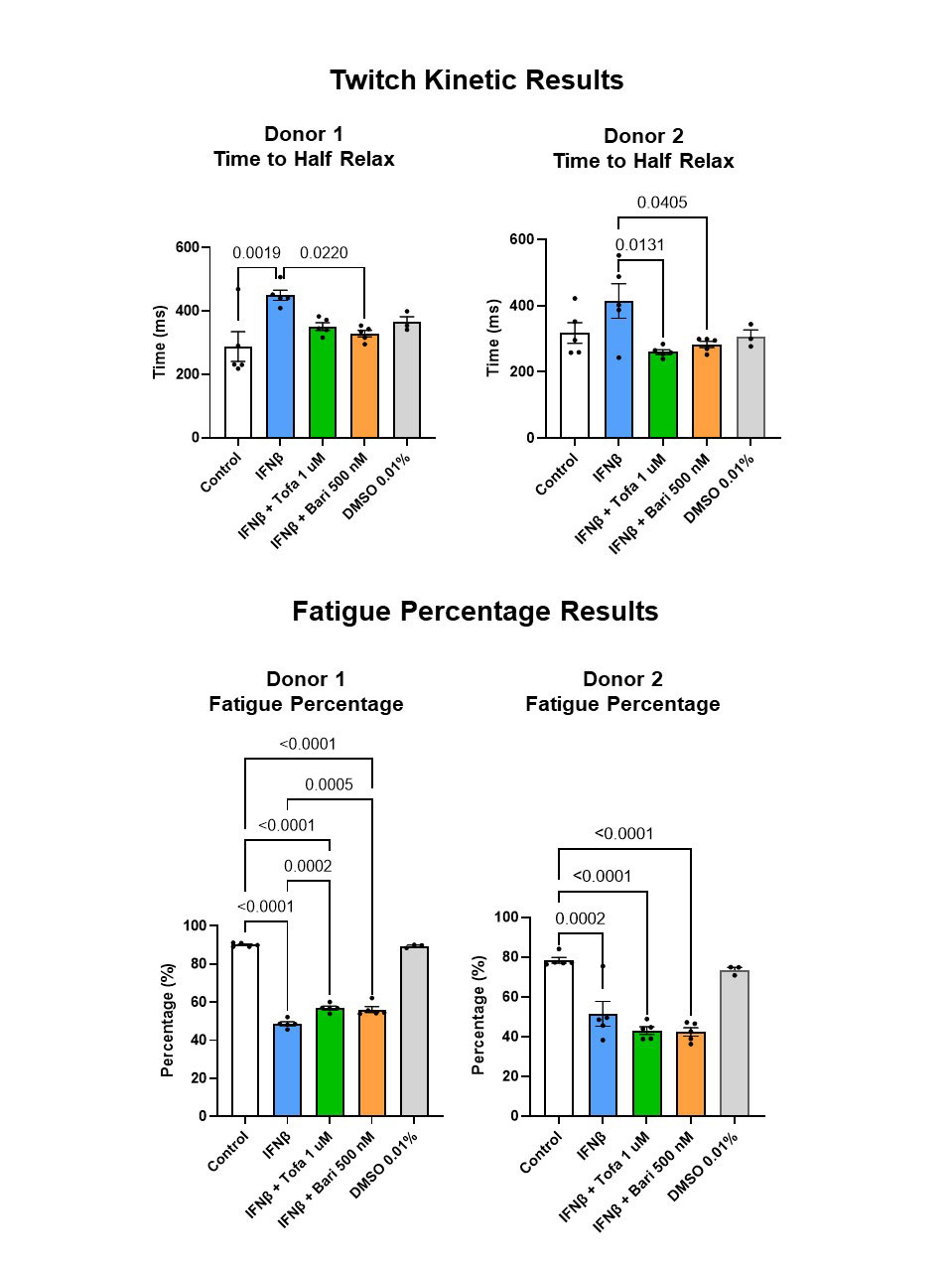
Results: Tofacitinib and baricitinib reversed IFNβ-associated twitch force reduction in one donor and significantly increased twitch force in the other donor (Fig. 2, top). IFNβ-associated reduction in tetanus force was reversed by tofacitinib in both donors and by baricitinib in one donor (Fig. 2, bottom). Time to maximum twitch force was increased in IFNβ-exposed myobundles in one donor (p< 0.0001). This increase normalized after tofacitnib and baricitinib. Twitch time to half-relax increased after IFNβ. This normalized after JAK inhibition (Fig. 3, top). IFNβ-associated decrease in fatigue did not reverse after tofacitinib or baricitinib treatment (Fig. 3, bottom).
Conclusion: JAK inhibitors tofacitinib and baricitinib reversed IFNβ-associated decrease in tetanus contractile force and slowed twitch kinetics but did not change muscle fatigue for 2 donors, suggesting donor-specific response. This 3D engineered tissue platform is a novel model to further develop for researching JDM pathogenesis and assessing therapeutic efficacy.
To cite this abstract in AMA style:
Covert L, Truskey G, Dvergsten J. Modeling Juvenile Dermatomyositis with Engineered Human Skeletal Muscle: Effects of Type I Interferonβ and Janus Kinase Inhibitors [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/modeling-juvenile-dermatomyositis-with-engineered-human-skeletal-muscle-effects-of-type-i-interferon%ce%b2-and-janus-kinase-inhibitors/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/modeling-juvenile-dermatomyositis-with-engineered-human-skeletal-muscle-effects-of-type-i-interferon%ce%b2-and-janus-kinase-inhibitors/