Session Information
Date: Tuesday, November 14, 2023
Title: Abstracts: Muscle Biology, Myositis & Myopathies – Basic & Clinical Science I
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Dermatomyositis (DM) and inclusion body myositis (IBM) are characterized by muscle weakness and inflammation, with emerging evidence of mitochondrial and neutrophil involvement. Prior work from our group has demonstrated the important role of mitochondrial-derived danger-associated molecular patterns to promote neutrophil activation in inflammatory conditions. In the current study, we aimed to investigate whether patients with myopathies had elevated levels of extracellular mitochondrial biomarkers promoting neutrophil-mediated inflammation.
Methods: Plasma samples were obtained from patients with inclusion body myositis (IBM, n=46), dermatomyositis (DM, n=40), and healthy individuals (HC, n=40) from Karolinska Institutet, Stockholm, Sweden; Johns Hopkins, Baltimore, USA; and University of Washington, Seattle, USA. DM patients were further categorized based on the presence of autoantibodies towards MDA5 (n= 19) and TIF1-gamma (n=21). Plasma levels of neutrophil-derived calprotectin and neutrophil elastase (NE)-DNA complexes, as well as mitochondrial-derived GDF-15, and N-formyl methionine (fMET), which are valuable indicators of potential mitochondrial driven-pathological processes, cellular stress, and/or tissue damage, were measured using ELISA. Statistical analysis was performed using GraphPad Prism 9.4.0.
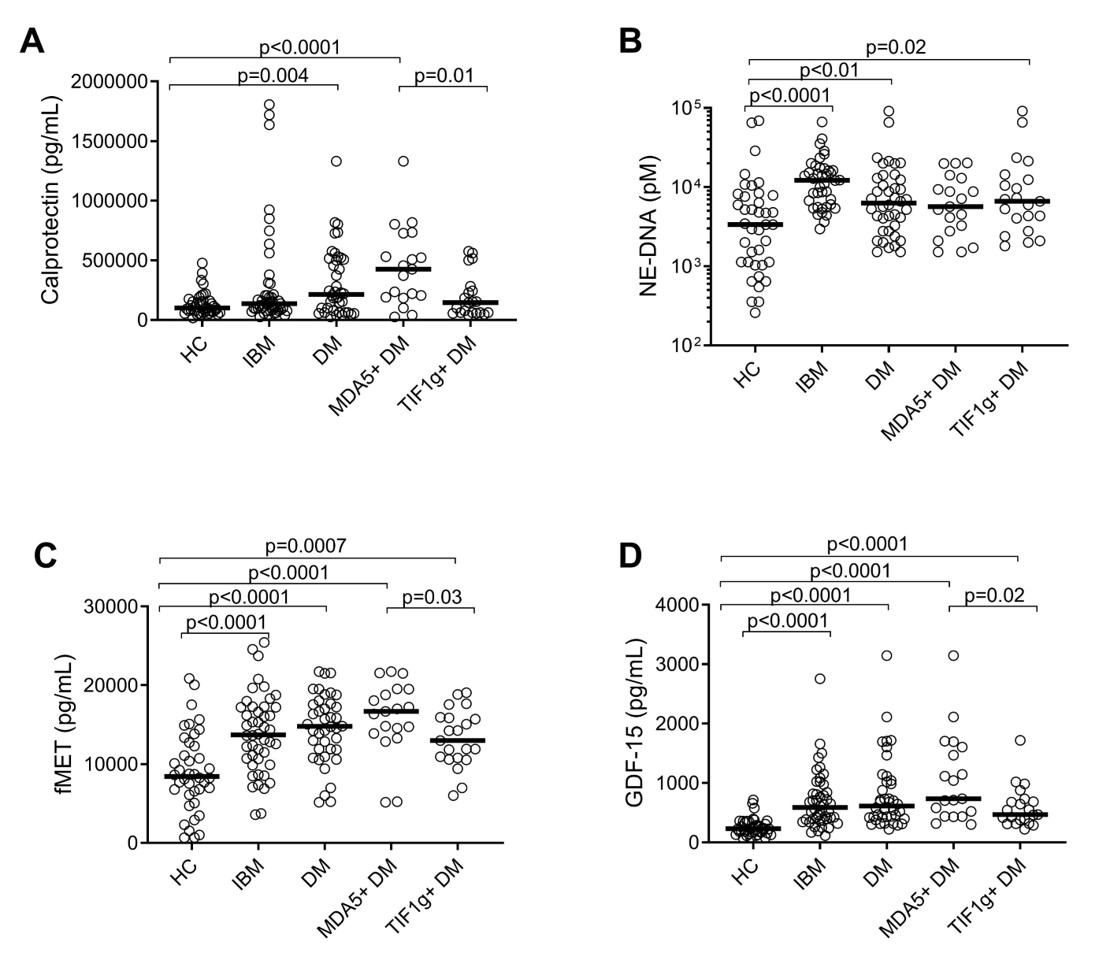
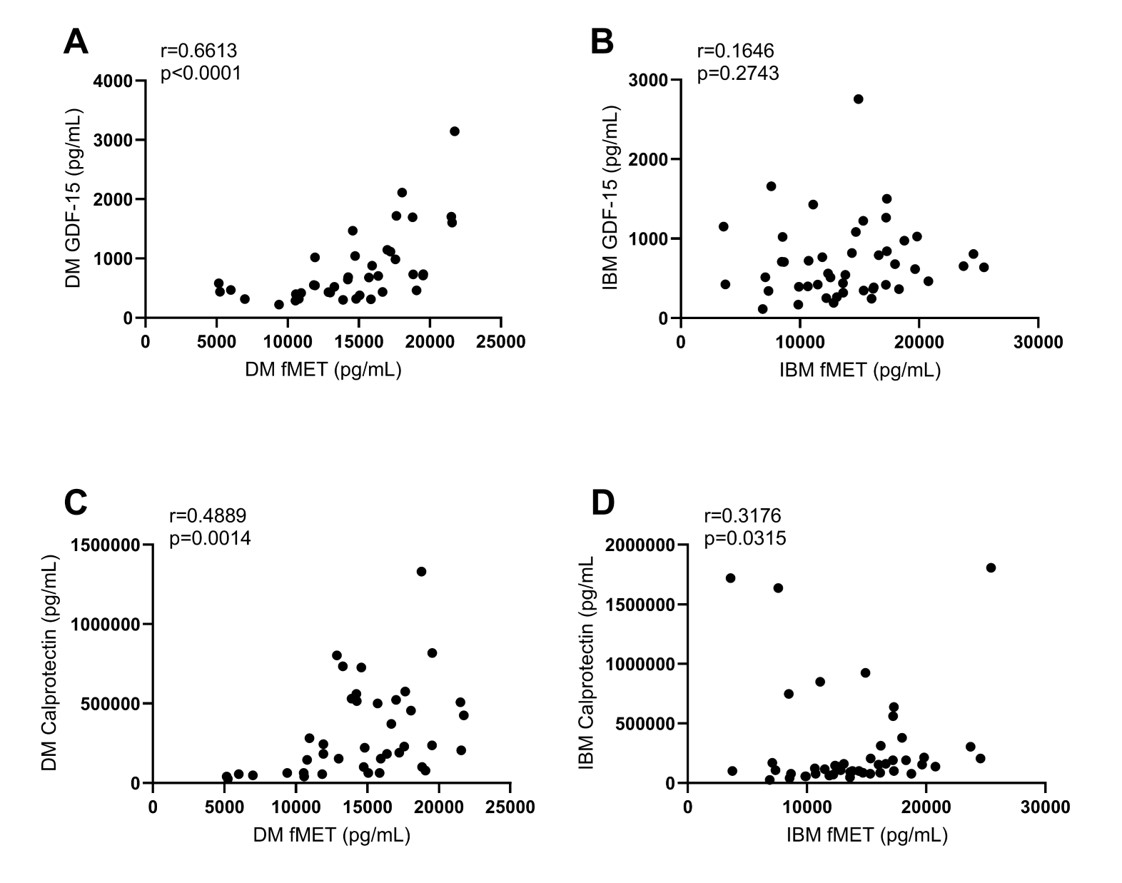
Results: Calprotectin levels were significantly elevated in DM patients, with the highest concentrations observed in those with MDA5 subtype (Figure 1A). Patients with IBM had similar levels of calprotectin as compared to HC. In contrast, levels of neutrophil extracellular traps, NETs (NE-DNA), were elevated in both DM and IBM patients, as compared to HC (Figure 1B). Both IBM and DM patients exhibited higher levels of mitochondrial-derived fMET and GDF-15 as compared to HC (Figures 1C and D), supporting a role for mitochondrial dysfunction and extrusion in these myopathies. Levels of GDF-15 and fMET were strongly correlated in DM but surprisingly no association was found in IBM (Figures 2A and B). Of note, consistent with other inflammatory conditions, levels of mitochondrial-derived fMET correlated strongly with neutrophil activation marker calprotectin in both IBM and DM (Figures 2C and D) suggesting that similar mechanisms, e.g. activation of neutrophils through fMET receptor FPR1, may operate in these subgroups of myopathies.
Conclusion: Our findings reveal distinct biomarker profiles in DM and IBM, highlighting the potential roles of mitochondria and neutrophils in myopathies. The differences and similarities observed between the subgroups suggest that calprotectin and NETs may play distinct roles in DM and IBM, while elevated levels of mitochondrial-derived fMET and GDF-15 indicate the potential involvement of mitochondrial dysfunction suggesting a common pathological mechanism. Further studies are warranted to determine the role of mitochondria and neutrophils in disease pathogenesis, as well as their clinical implications in monitoring disease activity.
Levels of A) Calprotectin, B) NE-DNA, C) fMET, and D) GDF_15 in patients with Inclusion Body Myositis (IBM), Dermatomyositis (DM), and Healthy Controls (HC). DM patients were subdivided into MDA5 (n=19) and TIF_1 gamma (n=21) subgroups. Mann-Whitney U test was used for statistical analyses.
Correlations between GDF_15 and fMET in A) DM and B) IBM patients. Correlations between calprotectin and fMET in C) DM and D) IBM patients.
Spearman’s correlation coefficient test.
To cite this abstract in AMA style:
Armando Gonzalez-Chapa J, Albayda J, Horuluoglu B, Michailidou D, Barguil Macedo M, Christopher-Stine L, Lundberg I, Lood C. Mitochondrial-Mediated Neutrophil Activation in Dermatomyositis (DM) and Inclusion Body Myositis (IBM): Insights into Pathogenesis and Therapeutic Implications [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/mitochondrial-mediated-neutrophil-activation-in-dermatomyositis-dm-and-inclusion-body-myositis-ibm-insights-into-pathogenesis-and-therapeutic-implications/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mitochondrial-mediated-neutrophil-activation-in-dermatomyositis-dm-and-inclusion-body-myositis-ibm-insights-into-pathogenesis-and-therapeutic-implications/