Session Information
Date: Monday, October 27, 2025
Title: (1088–1122) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Immune checkpoint inhibitors (ICIs) have transformed cancer therapy, but their use is often limited by immune-related adverse events (irAEs), particularly in barrier tissues such as the skin. The mechanisms underlying cutaneous irAEs remain incompletely understood. We aimed to identify cellular and antigenic drivers of skin irAEs in ICI-treated patients
Methods: We performed single-cell RNA sequencing (scRNA-seq) and T cell receptor (TCR) sequencing on paired lesional skin biopsies and peripheral blood mononuclear cells (PBMCs) from ICI-treated cancer patients who developed cutaneous irAEs. In parallel, we conducted ex vivo stimulation of patient PBMCs with microbial antigens, followed by flow cytometry to assess T cell activation and cytokine production
Results: Inflamed skin lesions harbored a dominant population of clonally expanded CD8⁺ T cells expressing cytotoxic effectors including GZMB, PRF1, and IFNG, indicative of tissue-damaging potential. Notably, CD8⁺ T cells from irAE lesions exhibited substantial expansion of the GZMB⁺ subset accompanied by a reduction in the less cytotoxic GZMK⁺ subset, highlighting a skew towards a more pathogenic cytotoxic phenotype. TCR clonotype tracking revealed overlap between skin-infiltrating clones and circulating CD8⁺ T cells, suggesting antigen-driven recruitment. Computational analysis using GILPH identified shared sequence motifs between patient TCRs and known anti-pathogen TCRs. Additionally, stimulation of patient PBMCs with Staphylococcus aureus and S. epidermidis antigens led to robust activation of GZMB⁺ CD8⁺ T cells and enhanced IFN-γ production
Conclusion: Our study identifies microbial antigens as candidate drivers of cytotoxic and inflammatory T cell responses in cutaneous irAEs. These findings highlight the contribution of microbial-reactive CD8⁺ T cells to irAE pathogenesis and suggest that targeting GZMB⁺ CD8⁺ effector functions may offer a therapeutic strategy to alleviate irAEs without compromising anti-tumor immunity
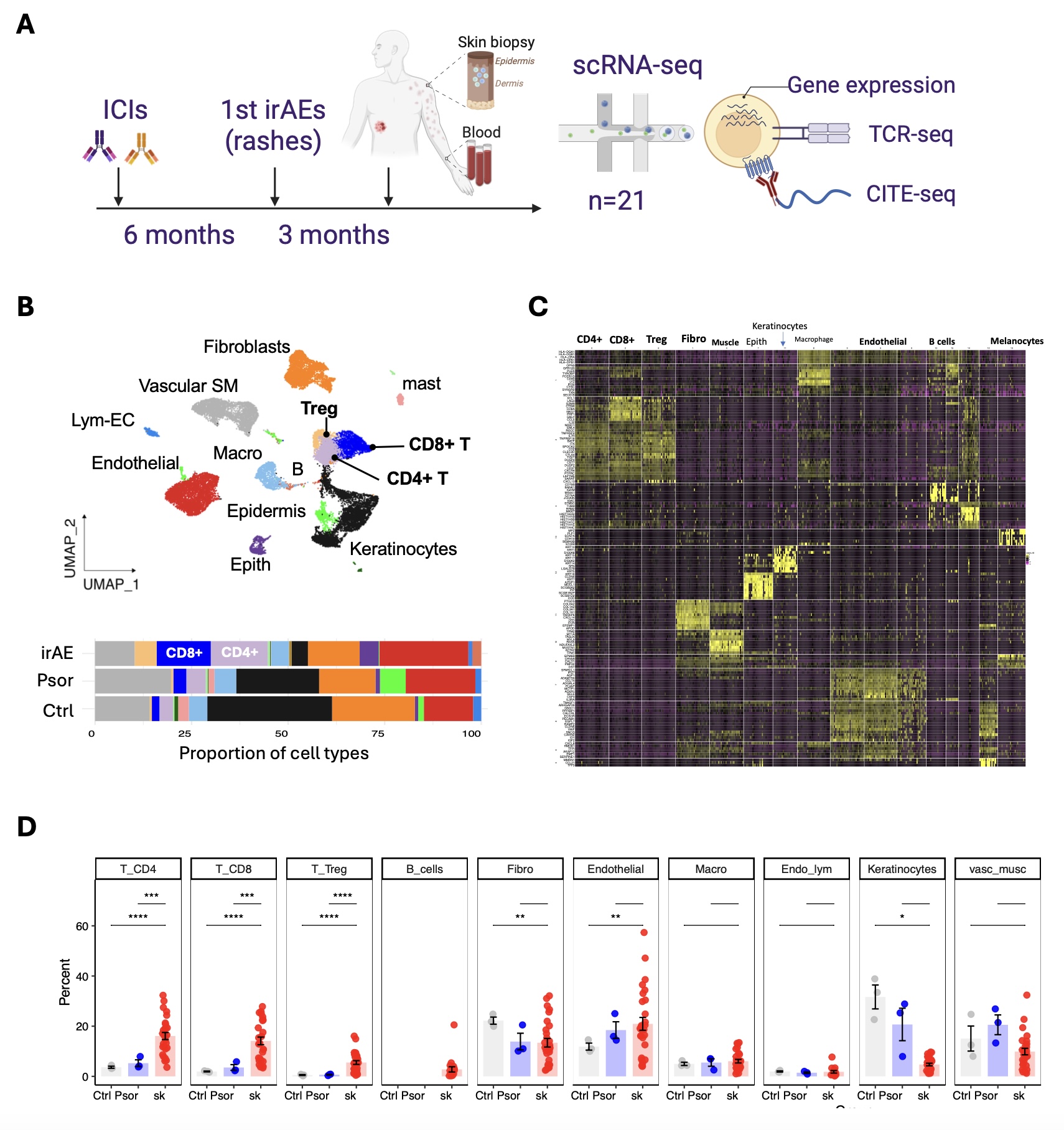 Figure 1. Single-cell analysis of skin and blood samples from patients with cutaneous irAEs following ICI therapy. (A) Schematic overview of the study design. Patients treated with immune checkpoint inhibitors (ICIs) developed immune-related adverse events (irAEs) manifesting as skin rashes. Skin biopsies and blood were collected approximately 3 months after rash onset (average 6 months post-ICI initiation) for single-cell RNA sequencing (scRNA-seq), TCR-seq, and CITE-seq. (B) UMAP embedding of skin immune and stromal cells from 28 samples, color-coded by annotated cell types. Bar plot (bottom) shows the proportion of each cell type across conditions: irAE (red), psoriasis (blue), and healthy control (gray), highlighting increased CD4⁺ and CD8⁺ T cell infiltration in irAE skin. (C) Heatmap of scaled gene expression across all clusters, showing top differentially expressed genes that distinguish immune, stromal, and epithelial populations. (D) Quantification of major immune and non-immune cell types across control (Ctrl), psoriasis (Psor), and irAE (sk) samples. Each dot represents an individual patient sample; statistical comparisons were performed using two-tailed t-tests. P < 0.05 (*), P< 0.01 (**), P< 0.001 (***), P< 0.0001 (****).
Figure 1. Single-cell analysis of skin and blood samples from patients with cutaneous irAEs following ICI therapy. (A) Schematic overview of the study design. Patients treated with immune checkpoint inhibitors (ICIs) developed immune-related adverse events (irAEs) manifesting as skin rashes. Skin biopsies and blood were collected approximately 3 months after rash onset (average 6 months post-ICI initiation) for single-cell RNA sequencing (scRNA-seq), TCR-seq, and CITE-seq. (B) UMAP embedding of skin immune and stromal cells from 28 samples, color-coded by annotated cell types. Bar plot (bottom) shows the proportion of each cell type across conditions: irAE (red), psoriasis (blue), and healthy control (gray), highlighting increased CD4⁺ and CD8⁺ T cell infiltration in irAE skin. (C) Heatmap of scaled gene expression across all clusters, showing top differentially expressed genes that distinguish immune, stromal, and epithelial populations. (D) Quantification of major immune and non-immune cell types across control (Ctrl), psoriasis (Psor), and irAE (sk) samples. Each dot represents an individual patient sample; statistical comparisons were performed using two-tailed t-tests. P < 0.05 (*), P< 0.01 (**), P< 0.001 (***), P< 0.0001 (****).
.jpg) Figure 2. Cytotoxic CD8⁺ T cells are expanded and activated in irAE skin lesions. (A) Volcano plot showing differentially expressed genes in CD8⁺ T cells from irAE versus psoriasis skin lesions. Cytotoxic effector genes (GNLY, GZMB, PRF1) and chemokine receptors (CXCR3, XCL1, CCL5) are significantly upregulated in irAE CD8⁺ T cells.
Figure 2. Cytotoxic CD8⁺ T cells are expanded and activated in irAE skin lesions. (A) Volcano plot showing differentially expressed genes in CD8⁺ T cells from irAE versus psoriasis skin lesions. Cytotoxic effector genes (GNLY, GZMB, PRF1) and chemokine receptors (CXCR3, XCL1, CCL5) are significantly upregulated in irAE CD8⁺ T cells.
(B) Violin plots showing expression of cytotoxic and chemotactic genes across CD8⁺ T cells from control (Ctrl), psoriasis (Psor), and irAE (sk) samples.
(C) UMAP plots depicting CD8⁺ T cell distribution and cytotoxicity scores across healthy, psoriasis, and irAE skin tissues.
.jpg) Figure 3. Bacterial stimulation induces activation of cytotoxic CD8⁺ T cell in PBMCs from irAE patients.
Figure 3. Bacterial stimulation induces activation of cytotoxic CD8⁺ T cell in PBMCs from irAE patients.
(A) Schematic of experimental workflow: PBMCs from irAE patients and healthy controls were stimulated ex vivo for 72 hours with S. aureus, S. epidermidis, or M. tuberculosis antigens. CD4⁺ and CD8⁺ T cell phenotypes were analyzed by flow cytometry (FACS). (B–C) Frequencies of CD4⁺ T cells (B) and CD8⁺ T cells (C) as a percentage of total lymphocytes across conditions.
To cite this abstract in AMA style:
Younis S, Acharya S, Swaminathan G, Wong H, Kim H, Eschholz A, Hegde S, McKnight A, Robinson W, Zaba L. Microbial activation of cytotoxic CD8⁺ T cells promotes skin immune-related adverse events in patients treated with immune checkpoint inhibitors [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/microbial-activation-of-cytotoxic-cd8%e2%81%ba-t-cells-promotes-skin-immune-related-adverse-events-in-patients-treated-with-immune-checkpoint-inhibitors/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/microbial-activation-of-cytotoxic-cd8%e2%81%ba-t-cells-promotes-skin-immune-related-adverse-events-in-patients-treated-with-immune-checkpoint-inhibitors/
