Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Memantine is an FDA proved drug utilized for the treatment of dementia and exerts its function by blocking the NMDA (N-methyl-D-aspartate) receptor, a calcium permeable ion channel that reduces cytotoxic calcium overload. Chondrocyte senescence is a crucial cellular event that contributes to articular cartilage degeneration during osteoarthritis (OA) development. However, little information has been reported regarding the effects of memantine and its downstream NMDA receptor on chondrocyte senescence and OA.
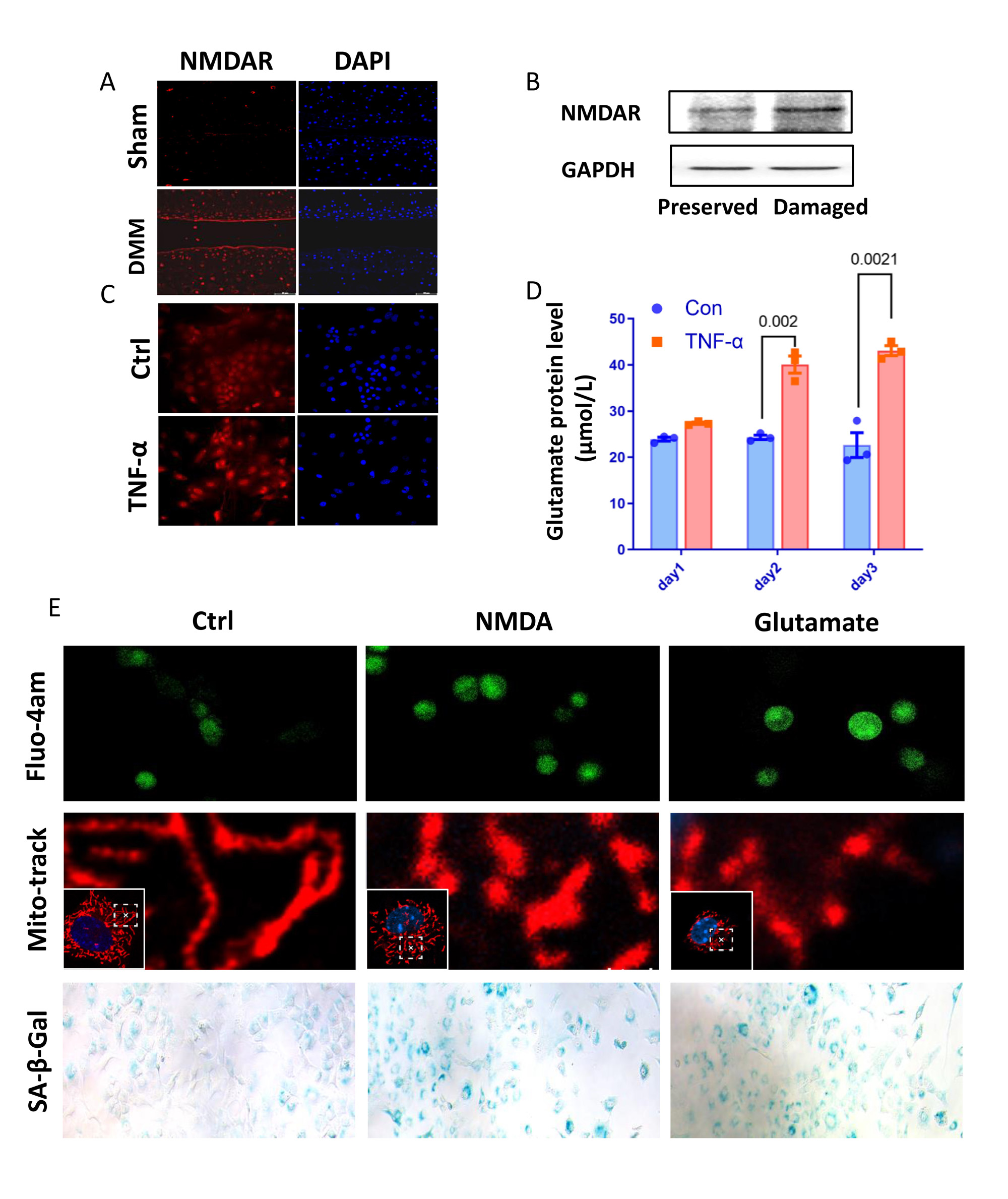
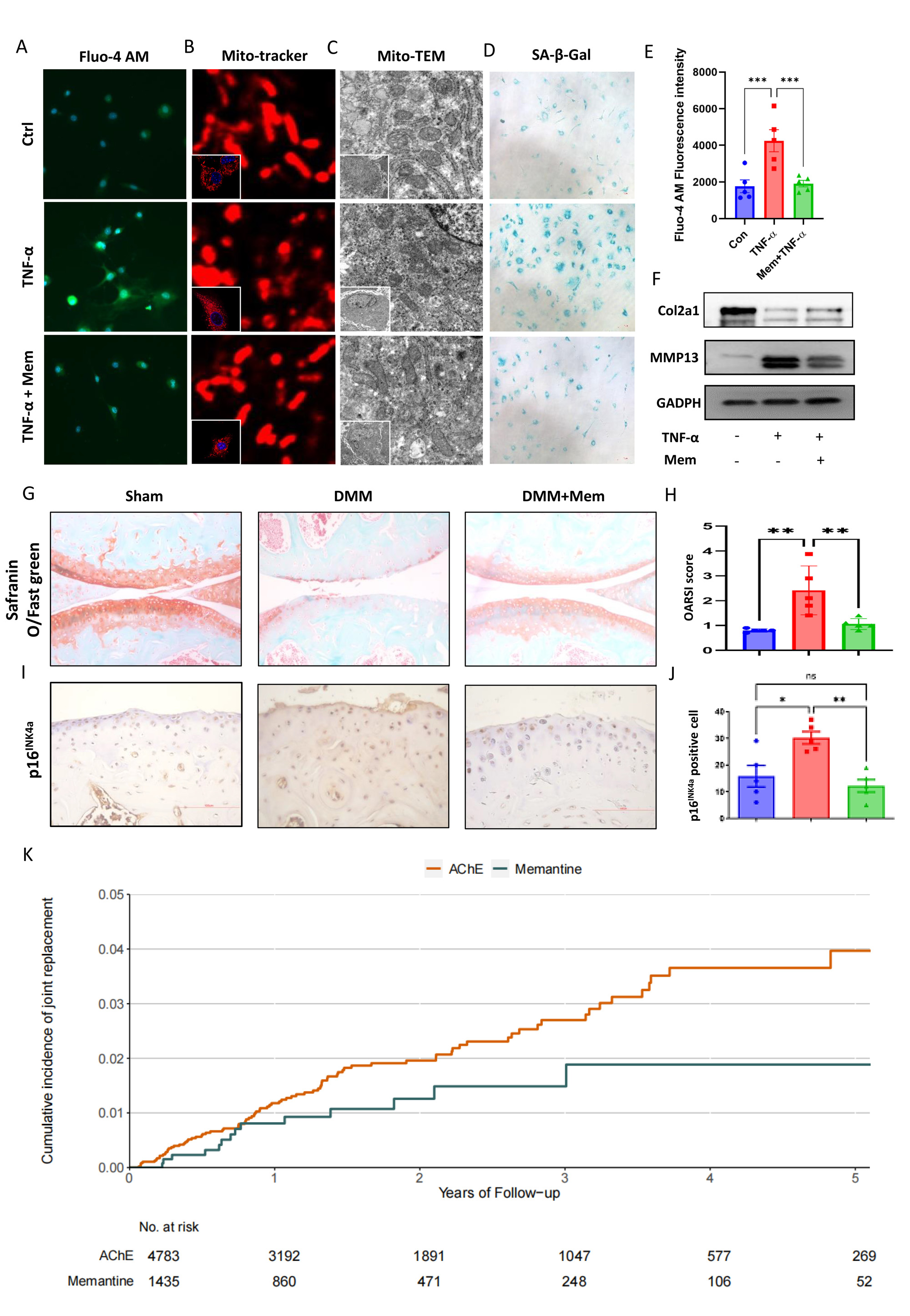
Methods: The protein levels of the NMDA receptor and its agonistic ligand, glutamate, were compared in OA and normal chondrocytes. The amount of intracellular calcium ions and mitochondrial damage were evaluated using specific fluorescent probes and transmission electron microscopy (TEM). Senescence-associated β-galactosidase (SA-β-gal) staining and p16INK4a were analyzed to assess chondrocyte senescence. The function of the NMDA receptor in chondrocyte senescence and OA were tested through agonists activation and gene knockout experiments. The therapeutic effects of memantine on OA were tested both in vitro and in vivo. Additionally, to verify the findings from animal samples, a propensity score matched human cohort study using the IQVIA Medical Research Data primary care database in the UK was conducted to compare the risk of OA associated joint replacement (the clinically relevant endpoint of OA) in memantine initiators with initiators of active comparator, i.e., acetylcholinesterase (AchE), among patients with dementia.
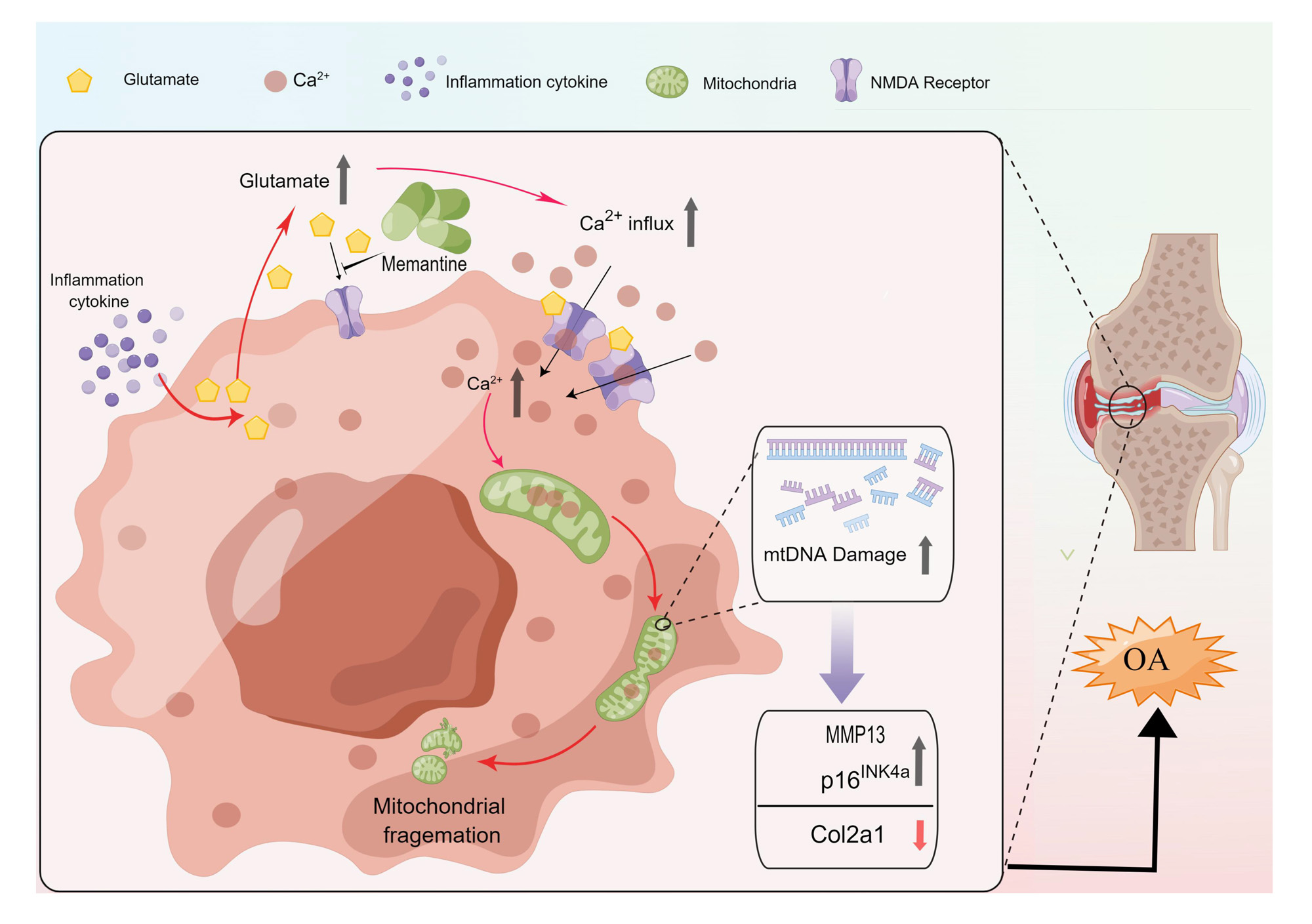
Results: The protein expression of the NMDA receptor and the secretion of glutamate were both significantly higher in OA chondrocytes. Activation of the NMDA receptor stimulated chondrocyte calcium overload, which resulted in mitochondrial fragmentation and chondrocyte senescence. Inhibiting the NMDA receptor by memantine and knockout NR1, the gene encoding NMDA receptor, resulted in reduced calcium influx, mitochondrial fragmentation as well as cellular senescence in OA chondrocyte. In addition, intra-articular injection of memantine in OA mice model exhibited protective effects on cartilage degeneration. What’s more, in the 1:5 propensity-score matched cohort study consisted of 6,218 patients (n=1,435 in memantine cohort; n=4,783 in AchE cohort), memantine initiators was associated with a lower risk of OA-associated joint replacement compared with AchE initiators (hazard ratio=0.57, 95% confidence interval: 0.34 to 0.99).
Conclusion: As a clinically licensed drug used for dementia, memantine shows promising therapeutic effects on OA. Mechanistically, it blocks NMDA receptor-mediated chondrocyte senescence. The protective effects of memantine on OA were verified not only through in vitro and in vivo experiments but also via a propensity-score matched human cohort study. These findings present robust evidence for repurposing memantine for the treatment of OA.
To cite this abstract in AMA style:
Wang N, Cheng Q, Li X, he k, Zhu J, Li H, Wei J. Memantine Attenuates the Development of Osteoarthritis by Blocking NMDA Receptor Mediated Calcium Overload and Chondrocyte Senescence [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/memantine-attenuates-the-development-of-osteoarthritis-by-blocking-nmda-receptor-mediated-calcium-overload-and-chondrocyte-senescence/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/memantine-attenuates-the-development-of-osteoarthritis-by-blocking-nmda-receptor-mediated-calcium-overload-and-chondrocyte-senescence/