Session Information
Session Type: Abstract Session
Session Time: 10:30AM-11:30AM
Background/Purpose: Force induced microdamage to joint tissue is hypothesized to trigger inflammatory events in the joint leading to arthritis. Patients with inflammatory arthritis, such as rheumatoid arthritis (RA) and spondyloarthritis (SpA), are found to have inflammation in “mechanical hotspots” and mechanical loading in mouse models of these diseases is pro-arthritic. To date, the molecular mechanism involved in converting force to a biological signal that promotes arthritis is unknown. This study aims to identify stretch induced genes in synovial fibroblasts, and the effect of these “mechano-sensitive” genes on arthritis.
Methods: Human synovial fibroblasts were stretched in vitro for 4hrs using the FlexCell system and analysed by microarray. Top stretch induced genes were measured in RA, SpA and healthy synovial tissue by qPCR. Patient synovium was further analysed by immunohistochemistry. Bhlhe40 knockout (KO) mice were subjected to collagen induced arthritis (CIA) and KBxN serum transfer induced arthritis (STIA). FACS was performed on ankle synovium, with synovial fibroblasts sorted for bulk RNAseq.
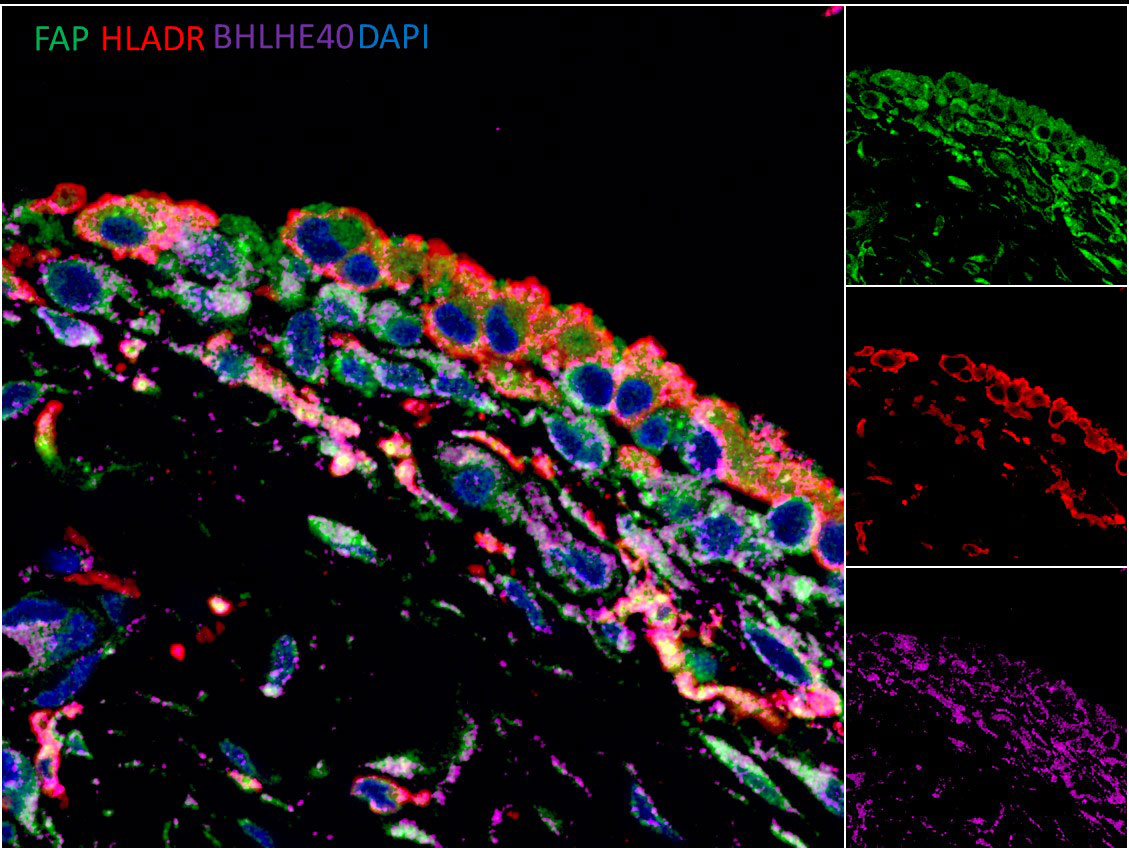
Results: 600 genes were found to be differentially expressed in stretched synovial fibroblasts (fold change > +/-1.5, adjusted p< 0.05). 25% of these genes were found to be transcription factors, which included BHLHE40. BHLHE40 mRNA was elevated in the synovial tissue of RA/SpA vs healthy subjects (1.56 fold change), and BHLHE40 protein was widely detectable in synovial fibroblasts and macrophages (Figure 1). Bhlhe40 KO mice were completely protected against CIA (incidence: 0% vs 40%, n=30 per group), but Bhlhe40 did not block the generation of anti-collagen antibodies. Bhlhe40 KO mice were partially protected against STIA (peak clinical score at day 7; 5.2 vs 6.8, n=15 per group), with reduced synovial macrophage (CD11b+Ly6G-F4/80+) and increased synovial fibroblast (CD45-CD31-PDPN+) frequency observed in the arthritic Bhlhe40 KO mice compared to wildtype (WT) controls. RNAseq revealed reduced inflammatory genes (Nos2, Lbp, Lcn2) and increased regulatory genes (Lgr5, Ereg) in Bhlhe40 KO vs WT fibroblasts from arthritic ankles.
Conclusion: BHLHE40 was identified as a force-induced gene in synovial fibroblasts and was found to be upregulated in patients with inflammatory arthritis. Importantly, Bhlhe40 strongly promotes joint inflammation in murine models of arthritis and uncouples systemic autoimmunity from joint tissue inflammation. Thus, we have identified BHLHE40 as a novel regulator of mechanical loading-associated inflammation.
To cite this abstract in AMA style:
Gracey E, Vlieghe C, Cambre I, Gilis E, Stappers F, Planckaert G, Lories R, Bozec A, Elewaut D. Mechanical Loading-induced BHLHE40 Promotes Inflammatory Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/mechanical-loading-induced-bhlhe40-promotes-inflammatory-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mechanical-loading-induced-bhlhe40-promotes-inflammatory-arthritis/