Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Rheumatoid arthritis (RA) is associated with an increased cardiovascular disease (CVD) burden, dramatically increasing the risk of mortality. Circulating antibodies to Malondialdehyde-Acetaldehyde (MAA) modified proteins have been found in patients with RA and CVD. MAA-modified protein adducts have also been identified in RA joint tissues, atherosclerotic lesions, and plaques. However, the proteins found in these tissues have been difficult to identify. Matrix gla protein (MGP) is a robust inhibitor of calcification, binds calcium, is a constituent of atherosclerotic lesions and is thought to play a pathogenic role in CVD. The purpose of this study to determine if MAA-modified MGP is present in both RA and CVD tissues.
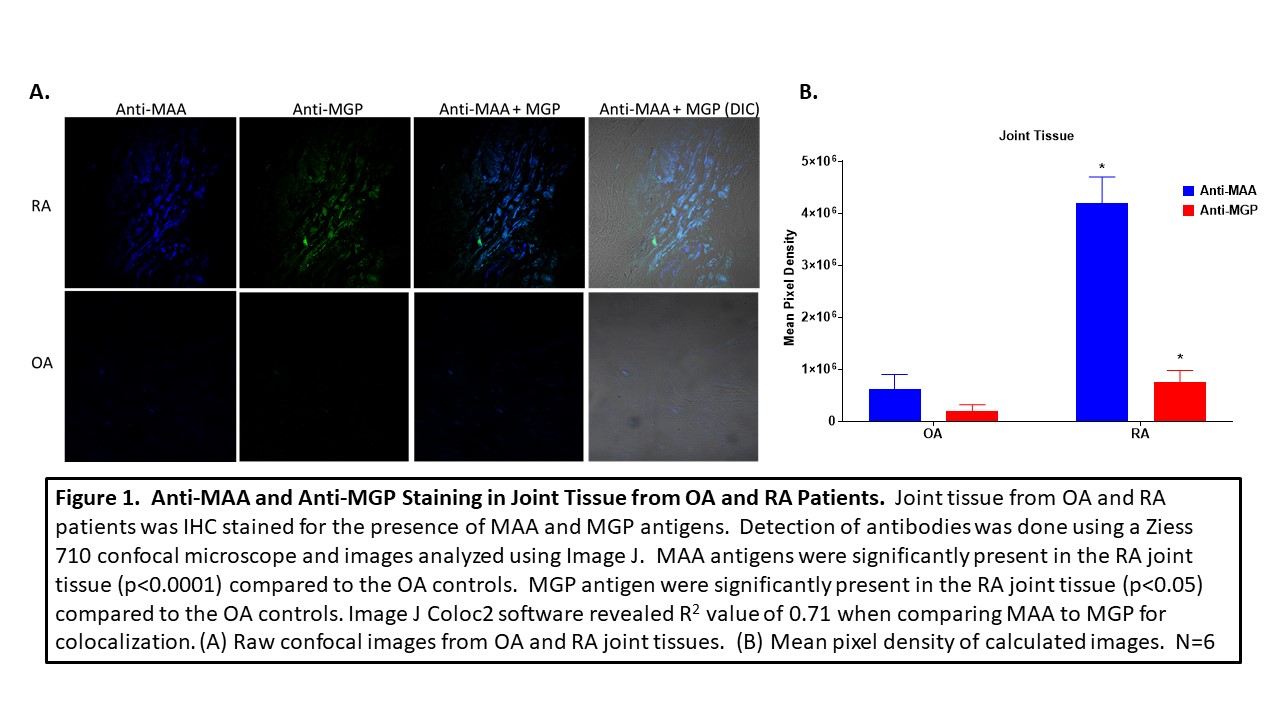
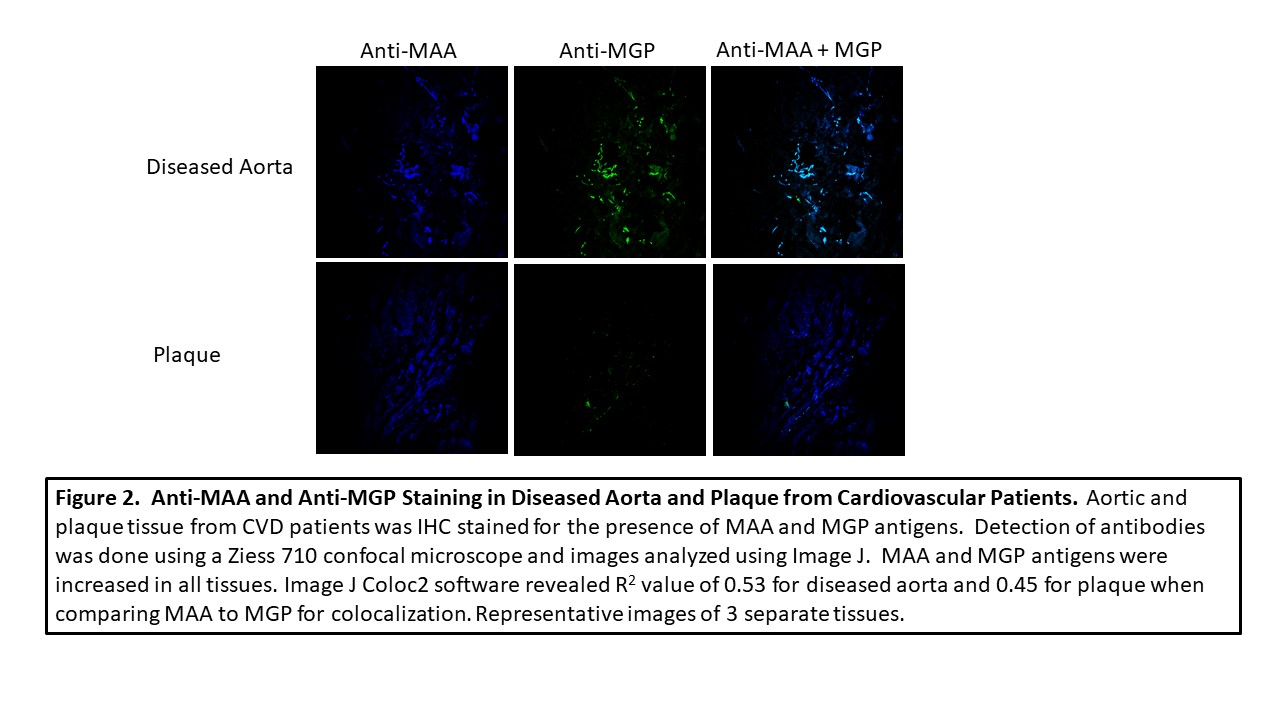
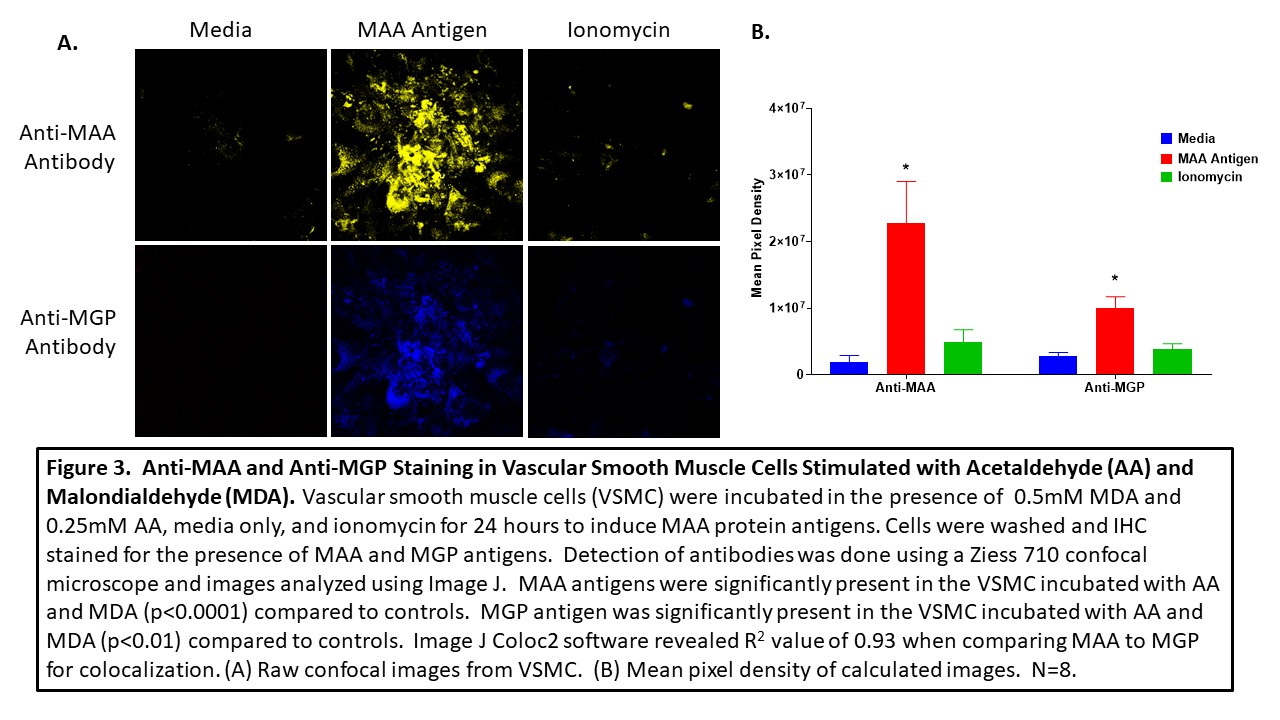
Methods: Joint tissues from 3 RA and 3 OA controls, along with 3 aortic lesions and 3 plaques obtained from patients with cardiovascular disease (CVD), were stained by immunohistochemistry (IHC) for the presence of MAA and MGP using zenon labeled 405 and 568 antibodies, respectively. Human vascular smooth muscle cells (VSMC) were incubated in the presence media, ionomycin, or MAA precursors, malondialdehyde (MDA; 0.5mM) and 0.25mM acetaldehyde (AA; 0.25mM), for 24 hours, fixed and stained for MGP and MAA using specific antibodies. A Ziess 710 confocal microscope was used to determine antibody reactivity and images analyzed using Image J for mean pixel density to quantitate the amount antibody present in tissue sections. For colocalization, Image J Coloc2 plug in was used to generate a correlation coefficient as means of quantifying the magnitude of overlapping colors.
Results: MAA expression was significantly increased in RA joint tissues (p< 0.0001) compared to joint tissues of OA controls. Likewise, MGP was expressed significantly more (p< 0.05) in RA joint tissues vs. OA controls (Figure 1). Co-localization of (r = 0.71) was present between MGP and MAA in the RA tissues. Although less than that seen in RA joint tissues, MGP was also co-localized with MAA in aortic lesions and plaques (r = 0.53; r = 0.45 Figure 2). Human VSMC incubated with MAA precursors demonstrated increased (p< 0.0001) expression of both MAA antigen and MGP (p< 0.01) compared to media or ionomycin controls (Figure 3) with evidence of strong MAA/MGP co-localization (r = 0.93).
Conclusion: Previous studies have shown that MAA-modified antigen characterizes diseased tissues in both RA and CVD. To date, however, identification of the precise proteins are MAA-modified in these lesions is unknown. Findings from this study suggest that MGP, a calcium binding protein, is present in diseased tissues in both RA and CVD and appears to be MAA adducted. Indeed, findings using human tissues were confirmed with in vitro experiments identifying MGP as a target for MAA modification. As MAA modification has been shown to alter the biologic function of other proteins, it is possible that MAA adduction could similarly alter MGP function, increasing calcium binding, causing tissue calcification, or impacting other calcium-dependent pathways. The presence of this unique protein modified with MAA in both RA and CVD tissue could be a potential link between the two diseases providing a mechanism as to why patients with RA experience a higher comorbidity with CVD.
To cite this abstract in AMA style:
Tieliwaerdi X, Aripova N, Duryee M, Jiang X, Klassen L, O'Dell J, England B, Anderson D, Mikuls T, Thiele G. Matrix Gla Protein (MGP) Modified with Malondialdehyde/Acetaldehyde Is Increased in Rheumatoid Arthritis and Cardiovascular Patients [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/matrix-gla-protein-mgp-modified-with-malondialdehyde-acetaldehyde-is-increased-in-rheumatoid-arthritis-and-cardiovascular-patients/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/matrix-gla-protein-mgp-modified-with-malondialdehyde-acetaldehyde-is-increased-in-rheumatoid-arthritis-and-cardiovascular-patients/