Session Information
Date: Tuesday, October 28, 2025
Title: (1780–1808) Osteoarthritis & Joint Biology – Basic Science Poster
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Protease cleavage of matrix proteins generates matrix fragments including fibronectin fragments (FN-f) found in OA cartilage and synovial fluid. Matrix fragments or “matrikines” activate signaling to increase production of OA mediators promoting a feed-forward loop of matrix destruction. Stimulation of chondrocytes with an Fn-f (FN7-10) that binds the α5β1 integrin has been shown to model the OA chondrocyte phenotype. Synovial fibroblasts contribute to OA by producing factors that promote synovitis and cartilage destruction. The purpose of this study was to determine if FN7-10 activates synovial fibroblasts to produce factors that promote monocyte migration, differentiation, and an OA chondrocyte phenotype.
Methods: OA synovial fibroblasts (SFs) were isolated from tissue obtained during knee replacement and chondrocytes from normal cartilage were from tissue donors. RNA and serum-free conditioned media were collected after 18 hours of stimulation of SFs with FN7-10 or PBS as control and used for RNAseq, cytokine/chemokine protein arrays for 80 proteins and Luminex assays designed to measure 18 OA mediators. To evaluate the effects of activated SFs on the OA microenvironment, a 4-hour FN7-10 stimulation was performed, followed by a transition to serum-free medium and overnight incubation. The conditioned media was harvested and assessed for its chemotactic effects on THP1 monocytes, and its impact on the expression of inflammatory mediators in THP1 monocytes and chondrocytes.
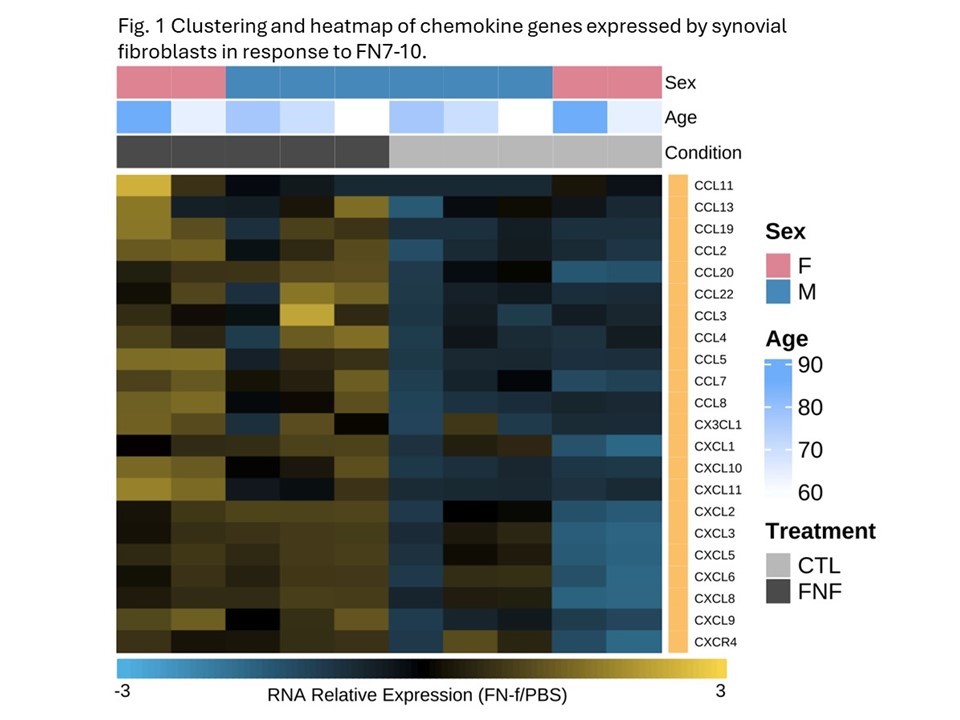
Results: SF RNAseq revealed 1487 differentially expressed (DE) genes (772 up and 715 down) in response to FN7-10 vs PBS control (DESeq2, adjusted p value < 0.05, absolute differences of log2 fold-change >1). Upregulated genes were enriched for KEGG pathways including TNF and IL-17 signaling, cytokine-cytokine receptor interaction, and NOD-like,Toll-like receptor, and NFκB signaling. There were 22 upregulated chemokine genes (Fig.1) many of which also exhibited increased protein levels (greater than 2-fold) as detected by cytokine/chemokine protein arrays and/or Luminex including IL-6, CXCL5, CCL5, MCP1-3, CXCL10, CXCL20, LIF, FGF-2, MMP-3, MMP-13 and TNFα. Conditioned media from SFs stimulated by FN7-10 induced monocyte migration, which was blocked by CCR2 and CXCR2 chemokine receptor inhibitors (Fig. 2), and induced macrophage differentiation. Chondrocytes incubated with SF FN7-10 conditioned media demonstrated significant increases in expression of OA mediators and a downregulation of aggrecan (ACAN) and COL2A1 (Table 1).
Conclusion: OA SFs produce proinflammatory cytokines, chemokines, and MMPs when exposed to a matrikine (FN7-10) produced by cartilage degradation. Chemokines released from activated SFs induce monocyte migration and promotion of a macrophage phenotype which could contribute to synovitis. SF factors also stimulated chondrocytes to produce key OA mediators, down regulate anabolic genes and increase expression of the OA biomarker CRTAC1. These findings demonstrate a mechanism for cross-talk between the synovium and cartilage mediated by cartilage matrix damage that could promote OA progression.
.jpg) Monocyte migration in response to synovial fibroblast conditioned media after FN7-10 stimulation. A). Serum-free conditioned media collected from OA synovial fibroblasts after treatment with FN7-10 or PBS as control was incubated with THP-1 cells in transwells. B). After 24hrs cells that had migrated out of the transwell to the well with conditioned media were counted (n=21 samples). C). THP-1 cells were pre-incubated with inhibitors to CCR2, CXCR2 or CXCR3 prior to placement in the transwells and treatment with the synovial fibroblast conditioned media. % migration was compared to cells without the receptor inhibitors set as 100%.
Monocyte migration in response to synovial fibroblast conditioned media after FN7-10 stimulation. A). Serum-free conditioned media collected from OA synovial fibroblasts after treatment with FN7-10 or PBS as control was incubated with THP-1 cells in transwells. B). After 24hrs cells that had migrated out of the transwell to the well with conditioned media were counted (n=21 samples). C). THP-1 cells were pre-incubated with inhibitors to CCR2, CXCR2 or CXCR3 prior to placement in the transwells and treatment with the synovial fibroblast conditioned media. % migration was compared to cells without the receptor inhibitors set as 100%.
.jpg) RNA was isolated from chondrocytes treated for 24hrs with synovial fibroblast (SF) conditioned media (CM) obtained from SFs treated for 24hrs with FN7-10 or PBS control. n=5 chondrocyte donor samples treated with n=3 samples of SF conditioned media. p value = FN7-10 CM vs PBS CM on Delta Ct values, 2 tailed, paired t test
RNA was isolated from chondrocytes treated for 24hrs with synovial fibroblast (SF) conditioned media (CM) obtained from SFs treated for 24hrs with FN7-10 or PBS control. n=5 chondrocyte donor samples treated with n=3 samples of SF conditioned media. p value = FN7-10 CM vs PBS CM on Delta Ct values, 2 tailed, paired t test
To cite this abstract in AMA style:
Loeser R, Fernandez Davila J, Coryell P, Chubinskaya S, Phanstiel D. Matrikine Activation of Synovial Fibroblasts Promotes Monocyte Migration and Stimulates an OA Chondrocyte Phenotype [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/matrikine-activation-of-synovial-fibroblasts-promotes-monocyte-migration-and-stimulates-an-oa-chondrocyte-phenotype/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/matrikine-activation-of-synovial-fibroblasts-promotes-monocyte-migration-and-stimulates-an-oa-chondrocyte-phenotype/