Session Information
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Cardiovascular disease (CVD) remains the leading cause of death in patients with rheumatoid arthritis (RA). General population CVD risk calculators underestimate risk in RA, even when accounting for RA disease activity. To inform CVD risk calculator development in RA, we tested the hypothesis that molecular phenotypes composed of cytokines, chemokines, autoantibodies, and matrix metalloproteinases (MMPs) would predict incident CVD including major adverse cardiac events (MACE) and heart failure (HF).
Methods: In a multicenter, prospective cohort of US veterans with RA, we followed participants without prevalent CVD from enrollment to MACE (defined as myocardial infarction, coronary revascularization, stroke, or CVD death), HF hospitalization, death, or end of study period (12/2020). From banked serum collected at enrollment, we measured 33 cytokines and chemokines (MesoScale platform), MMPs (MMP-1, -3, -7, -9; MesoScale), RF (nephelometry), anti-CCP (2nd generation ELISA), and anti-malondialdehyde acetaldehyde (MAA) antibody concentrations (IgA, IgM, and IgG anti-MAA-albumin, anti-MAA-collagen, and anti-MAA-vimentin; ELISA). Using validated algorithms, we identified MACE and HF outcomes using National VA data linked with the National Death Index. Analyte data were log-transformed and standardized (per 1 SD). Principal component analysis (PCA, an unsupervised machine learning approach) was performed, retaining components with Eigenvalues >1. Associations of principal component (PC) scores with a composite CVD outcome (MACE or HF), as well as MACE, individual MACE components, and HF were assessed using multivariable Cox regression models, adjusting for age, sex, race, smoking status, and RA disease activity (DAS28).
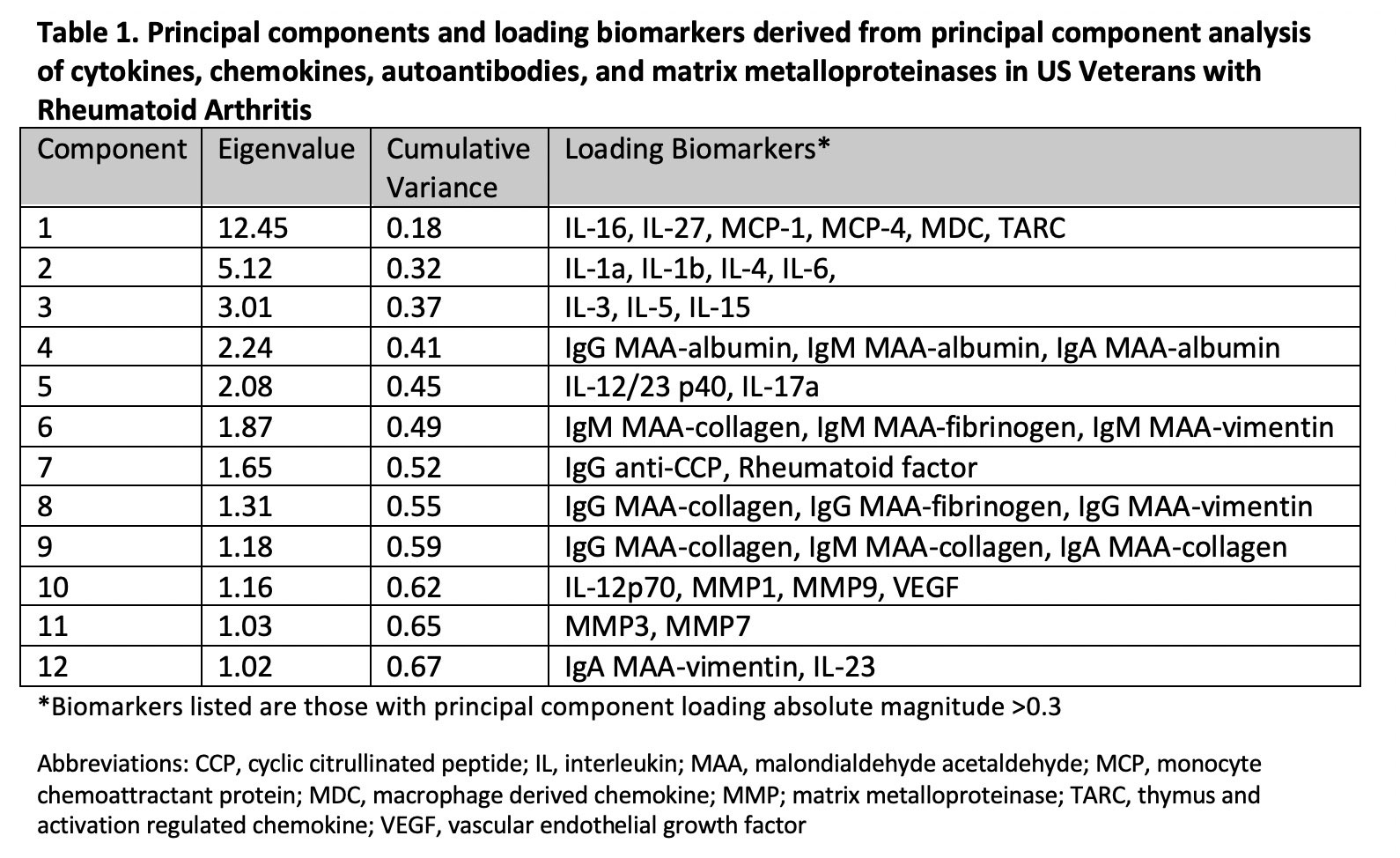
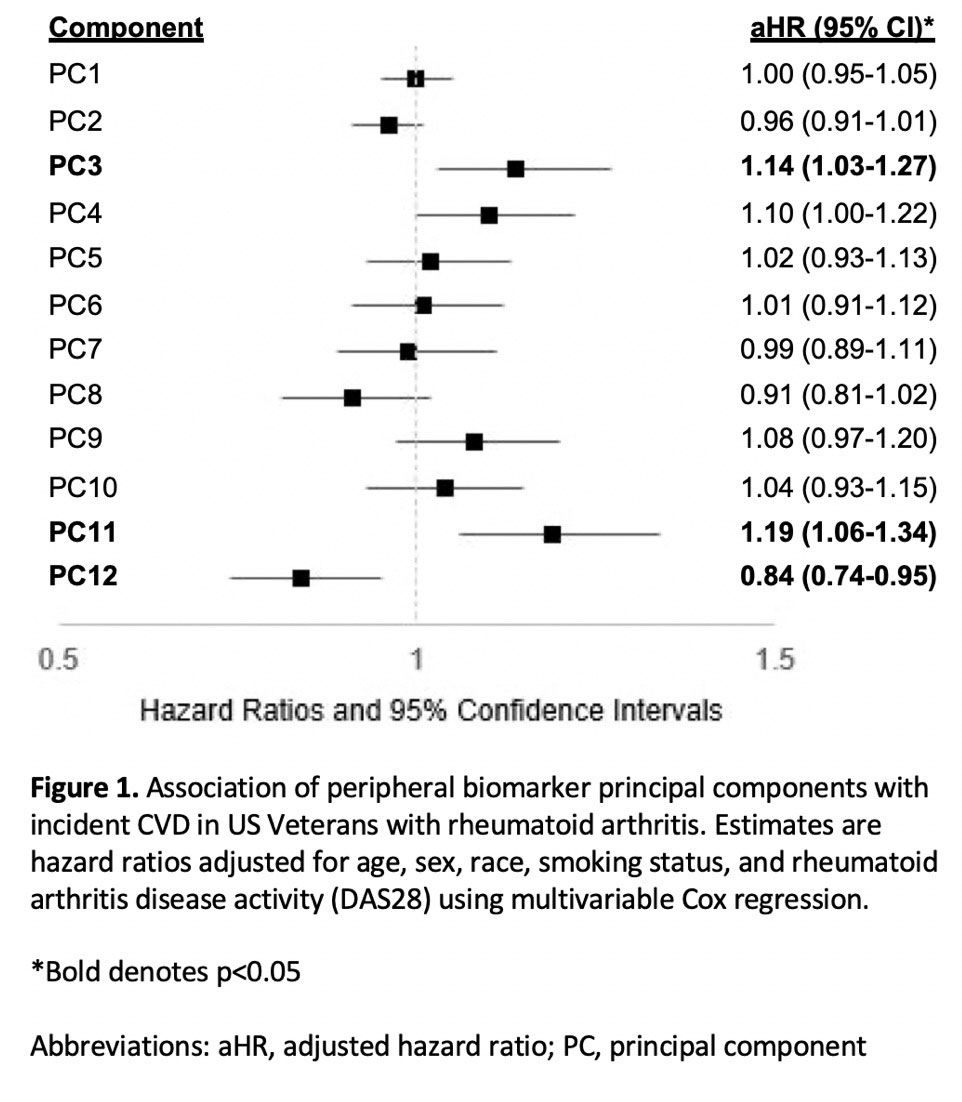
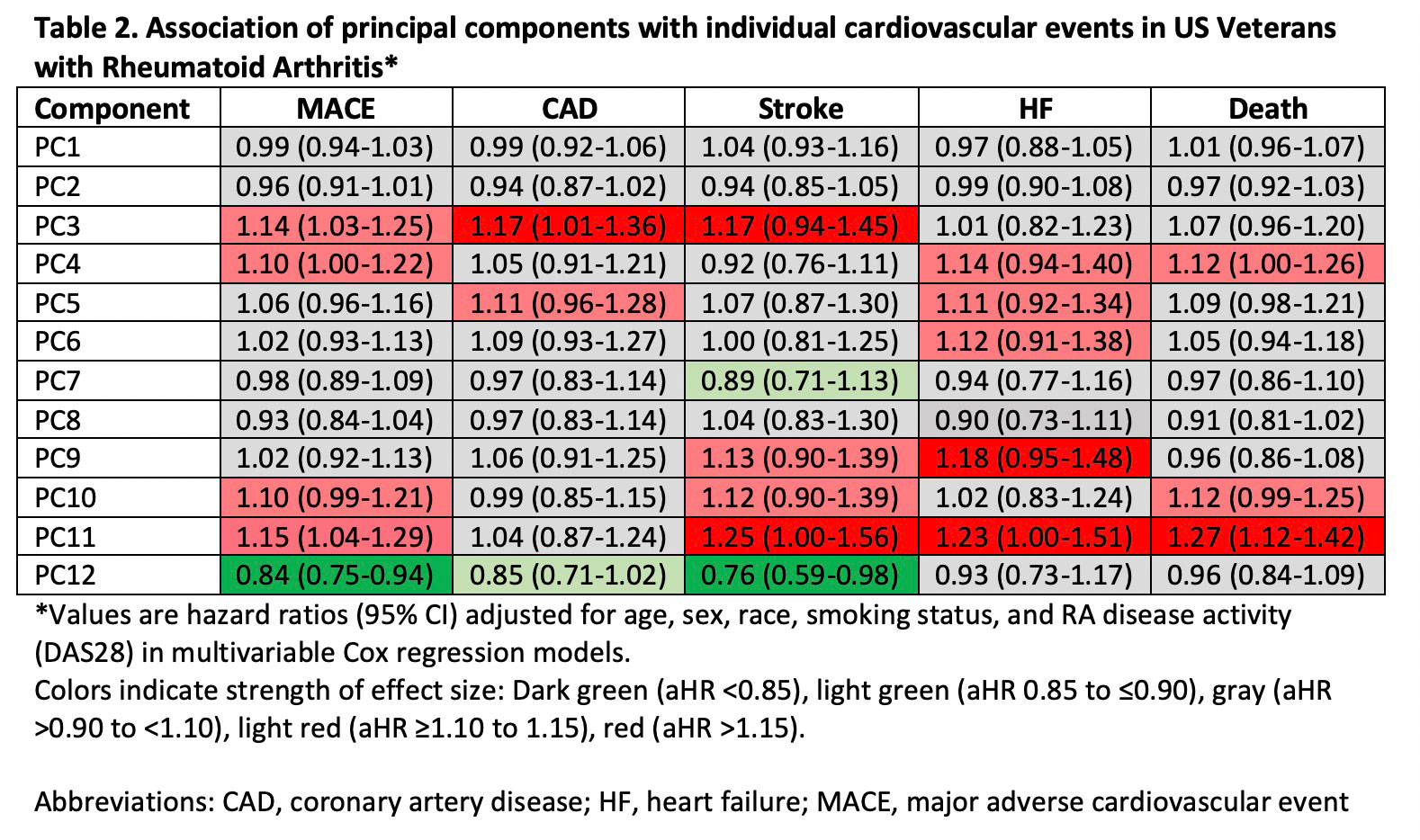
Results: Among 2,370 patients (mean age 64 years, 87% male, mean DAS28 3.7) without prior MACE or HF, we observed 316 composite CVD events over a mean follow up of 7.8 years. PCA yielded 12 unique molecular profiles from 51 total analytes (Table 1). PC3 (represented by IL-3, -5, -15; adjusted hazard ratio [aHR] 1.14, 95% CI 1.03-1.27) and PC11 (MMP-3, -7; aHR 1.19, 95% CI 1.06-1.34) scores were most strongly associated with higher CVD risk, while PC 12 scores (IgA MAA-vimentin and IL-23; aHR 0.84, 0.74-0.95) were negatively associated with CVD risk (Figure 1). PCs characterized by pro-inflammatory cytokines were most closely associated with MACE events (aHR 1.17, 95% CI 1.01-1.36), while those characterized by MMPs were most strongly associated with HF, stroke, and CVD-related death (range aHR 1.23-1.27) (Table 2).
Conclusion: In a multicenter RA cohort, principal component analysis of cytokines, chemokines, MMPs, and RA-related autoantibodies yielded molecular profiles predictive of incident CVD, independent of RA disease activity. These findings show the potential for comprehensive biomarker assessment and molecular profiling to inform CVD risk stratification in RA across different CVD event types. Continued research is needed to determine whether these profiles improve risk prediction beyond established CVD risk calculators to support targeted CVD risk management in patients with RA.
To cite this abstract in AMA style:
Johnson T, Gundry R, Lindsey M, Roul P, Yang Y, Baker J, Sauer B, Cannon G, Thiele G, Mikuls T, England B. Machine Learning Identifies Molecular Phenotypes That Predict Incident Cardiovascular Disease in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/machine-learning-identifies-molecular-phenotypes-that-predict-incident-cardiovascular-disease-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/machine-learning-identifies-molecular-phenotypes-that-predict-incident-cardiovascular-disease-in-patients-with-rheumatoid-arthritis/