Session Information
Date: Saturday, November 12, 2022
Title: Abstracts: Innate Immunity
Session Type: Abstract Session
Session Time: 5:00PM-6:00PM
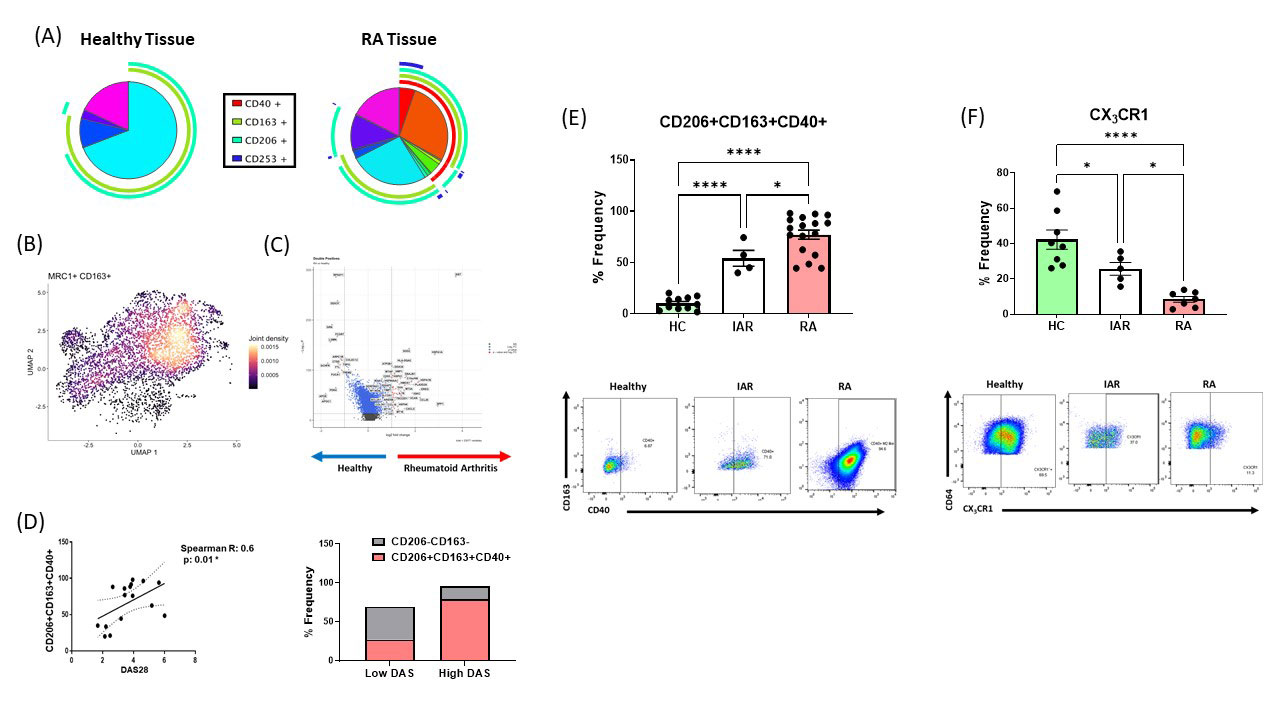
Background/Purpose: Synovial-tissue macrophages significantly contribute to Rheumatoid Arthritis, yet the precise nature/function of macrophage subsets within the inflamed joint remains unexplored. Here we explore the spectrum of distinct macrophage activation states residing within the synovium of RA, at risk and healthy individuals.
Methods: Single-cell synovial-tissue suspensions from RA(n=44), IAR(n=5), HC(n=11) were obtained, synovial macrophage subsets examined by advanced flow-cytometric analysis, single-cell/bulk RNA-sequencing, metabolic and functional assays.
Results: Multidimensional analysis identifies enrichment of CD206+CD163+ synovial-tissue macrophages co-expressing CD40 in RA compared to healthy synovial-tissue, with frequency of CD206+CD163+CD40+ macrophages associated with increased disease activity and treatment response. In contrast, CX3CR1-expressing macrophages enriched in healthy synovium are significantly depleted in RA. Importantly this signature of enriched CD40 expression coupled with depleted CX3CR1 expression is an early phenomenon, occurring prior to clinical manifestation of disease in individuals ‘at-risk’ of RA (IAR). In-depth RNAseq and metabolic profiling of sorted RA synovial-macrophages identified that this population is transcriptionally distinct, displaying unique inflammatory, phagocytic and tissue-resident gene signatures, paralleled by a bioenergetically stable profile as indicated by NAD(P)H emission. Functionally CD206+CD163+ RA macrophages are potent producers of pro-inflammatory mediators (reversed by CD40-signalling inhibition) and induce an invasive phenotype in healthy synovial-fibroblasts. These findings identify a distinct pathogenic population of synovial-tissue macrophage involved in shaping the immune response in RA.
Conclusion: We have identified a novel population of tissue-resident macrophages in the RA synovium which are transcriptionally/metabolically distinct and capable of contributing to disease pathology. Crucially, this signature is present pre-disease representing a unique opportunity for early diagnosis and therapeutic intervention.
To cite this abstract in AMA style:
Hanlon M, Canavan M, Smith c, Floudas A, Neto N, Song Q, Gallagher P, Mullan R, Hurson C, Moran B, Monaghan M, Nagpal S, Veale D, Fearon U. Loss of Synovial Tissue Macrophage Homeostasis Precedes Rheumatoid Arthritis Clinical Onset [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/loss-of-synovial-tissue-macrophage-homeostasis-precedes-rheumatoid-arthritis-clinical-onset/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/loss-of-synovial-tissue-macrophage-homeostasis-precedes-rheumatoid-arthritis-clinical-onset/