Session Information
Date: Monday, November 14, 2022
Title: RA – Treatment Poster IV
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Elderly patients with rheumatoid arthritis (RA) tend to have a higher risk for drug-related adverse events (AEs) than younger patients, and the effectiveness and safety of the treat-to-target (T2T) approach for patients with elderly-onset RA (EORA) are unclear. We have conducted a prospective cohort study, and reported that the three-year achievement rates of simplified disease activity index (SDAI) remission and health assessment questionnaire disability index (HAQ-DI) ≤ 0.5 were significantly higher in EORA patients adhering to T2T than those not adhering to T2T (1). Furthermore, multivariable analysis showed chronic lung diseases (CLD) at baseline were associated with increased SDAI after implementation of T2T (2) and serious AEs (SAEs) within the first three years (1). In this study, we evaluated the five-year effectiveness and safety of the T2T strategy in patients with EORA and CLD at baseline.
Methods: Patients who were methotrexate (MTX)-naïve EORA and had moderate-to-high disease activity based on a 28-joint disease activity score using erythrocyte sedimentation rate were registered in the study from 2008 to 2015. Patients with poor prognostic factors started MTX, while tacrolimus was started in patients who had difficulty receiving MTX due to interstitial lung disease (ILD). Glucocorticoids (GCs) were started at the discretion of an attending physician. If patients did not achieve low disease activity at Week 24 or later, a tumor necrosis factor inhibitor (TNFi) was started. If the first TNFi was ineffective, it was switched to another biological disease-modifying antirheumatic drug (bDMARD). The primary outcomes were a proportion of patients achieving SDAI remission and HAQ-DI ≤0.5 at Year 5 by non-responder imputation (NRI). Secondary outcomes were a proportion of patients with drop-out and SAEs at Year 5. Drop-out was defined as lost-to-follow-up or deterioration of RA-associated extra-articular disease or other rheumatic disease requiring moderate to high dose GC. SAEs included adverse events requiring hospitalization and death.
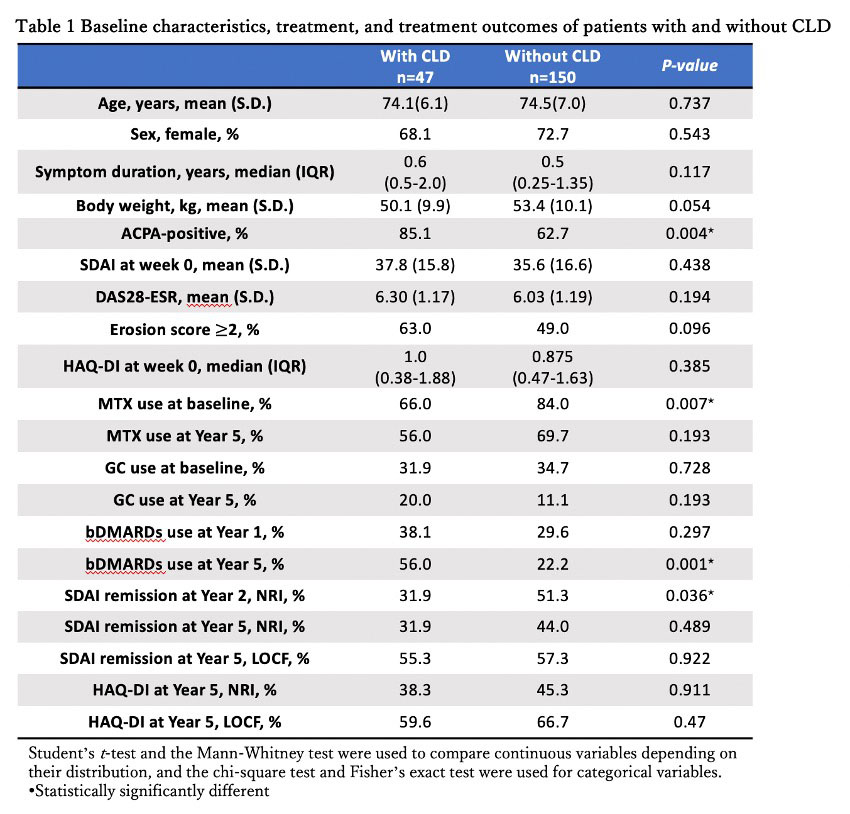
Results: Among 197 patients with EORA, 47 patients had CLD (ILD, n=31; airway disease, n=12; emphysema, n=11; others, n=2) (Table 1). The proportion of MTX use at baseline was significantly lower in patients with CLD than those without, but there was no significant difference between the groups at Year 5. The proportion of bDMARDs use was higher in patients with CLD than those without at Year 5 (Table 1). There was no significant difference between the groups in SDAI remission and HAQ-DI ≤ 0.5 at Year 5 (Table 1). The drop-out rate at Year 5 was not significantly different between the two groups. The cumulative probability of SAEs was 73.6% and 36.6% in those with and without CLD, respectively (p < 0.001).
Conclusion: EORA patients with CLD at baseline had a higher probability of SAEs, but the long-term treatment outcomes were not different between the two groups. T2T is effective and tolerated even among high-risk patients with EORA and CLD.
References
1. Sugihara T, et al. Rheumatology (Oxford). 2021;60(9):4252-4261
2. Sugihara T, et al. EULAR 2022 (POS0522)
To cite this abstract in AMA style:
Nomura M, Sugihara T, Baba H, Ishizaki T, Hosoya T, Kamiya M, Matsumoto T, Kubo K, Hirano F, Kojima M, Miyasaka N, Yasuda S, Harigai M. Long-term Outcome of a Treat-to-target Strategy in Elderly-onset Rheumatoid Arthritis with Chronic Lung Diseases [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/long-term-outcome-of-a-treat-to-target-strategy-in-elderly-onset-rheumatoid-arthritis-with-chronic-lung-diseases/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-outcome-of-a-treat-to-target-strategy-in-elderly-onset-rheumatoid-arthritis-with-chronic-lung-diseases/