Session Information
Date: Sunday, November 7, 2021
Title: Pediatric Rheumatology – Clinical Poster II: SLE, JDM, & Juvenile Scleroderma (0764–0785)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
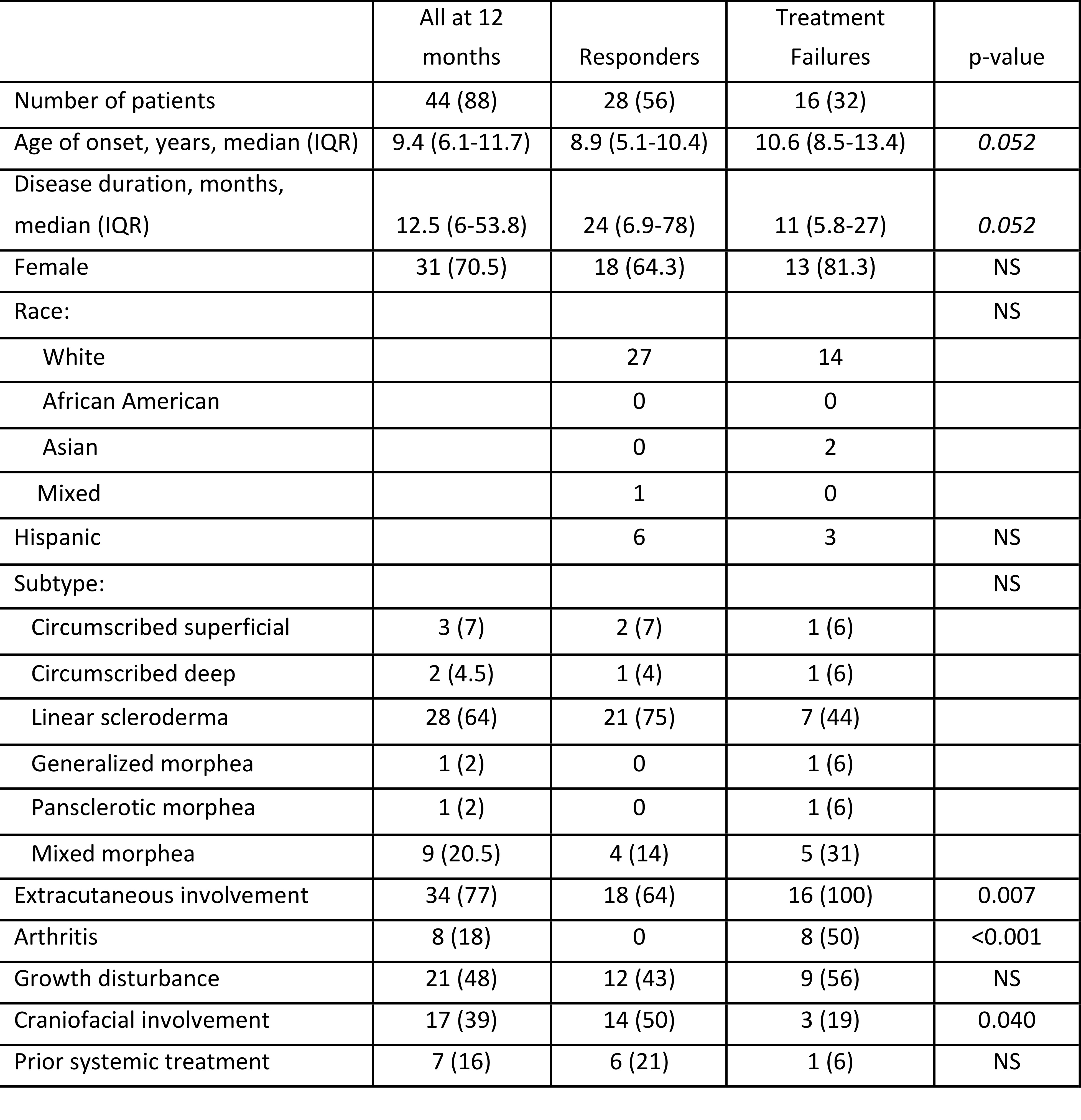
Background/Purpose: Juvenile localized scleroderma (jLS) is a rare chronic inflammatory and fibrosing disease associated with a high risk for morbidity in children. Methotrexate (MTX) has been identified as effective treatment, but data is limited as to the optimal duration, and need for corticosteroid (CS) treatment. The LS group of the Childhood Arthritis and Rheumatology Research Alliance (CARRA) developed standardized regimens (consensus treatment plans, CTPs) for comparative effectiveness studies. The results of a 1-year follow-up of a pilot multi-center study of treating 50 patients with a MTX-based CTP were previously reported. Of the 44 (88%) patients that completed 1 year of follow-up, 33 (66%) were rated as responders and 11 (22%) as non-responders. We now report on the long-term follow-up of these patients.
Methods: Patients enrolled in the pilot CTP study were eligible to enroll in the long-term extension, with study visits completed at 24 and 36 months. Each patient was evaluated by the same investigator for all 3 years of the study, using the same set of standardized clinical outcome measures for all visits. Treatments and adverse events that occurred since the last study visit were collected. Descriptive analysis was performed of all patients that had completed at least 12 months of follow-up, with p values calculated by Z-score or Mann Whitney U test. Inactive disease was defined as physician global assessment of activity (PGA-A) = 0, remission off medicine as PGA-A = 0 with the patient off treatment, and treatment failure as active disease requiring additional medication besides that specified in CTP. Patients who experienced treatment failures at any time during the study were compared to patients who were never treatment failures.
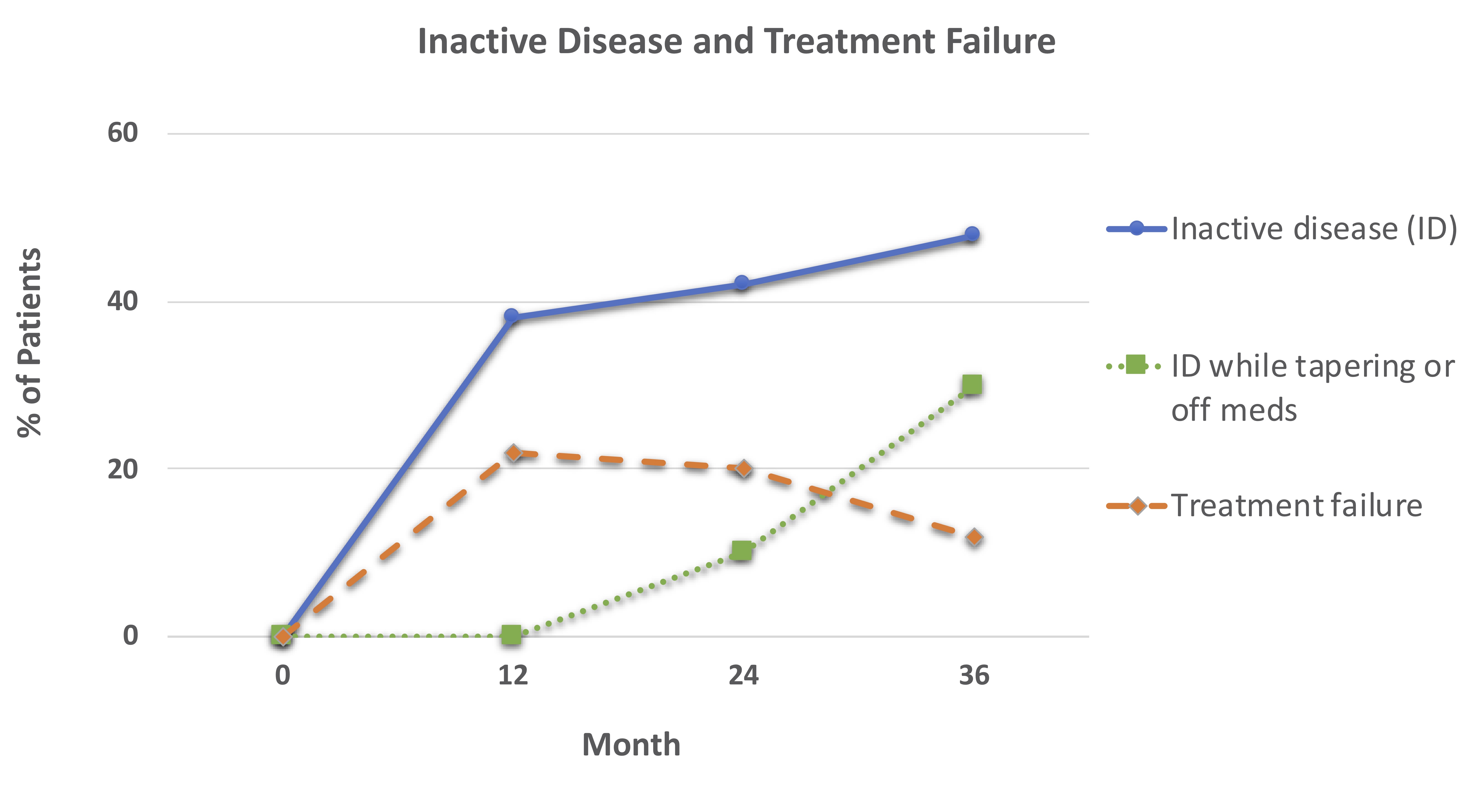
Results: Most patients were female (70%) and had linear scleroderma (Table). Thirty-seven (74%) of patients completed 36 months of follow-up (Figure 1). Over time, more patients achieved inactive disease, with 30% able to maintain inactive disease while tapering or discontinuing methotrexate (Figure 1). Treatment failures (TF) occurred most commonly in years 1 and 2 when ~20% of patients were non-responders. In addition to 11 patients who experienced TF in the first 12 months, 5 additional patients experienced TF by 24 months.
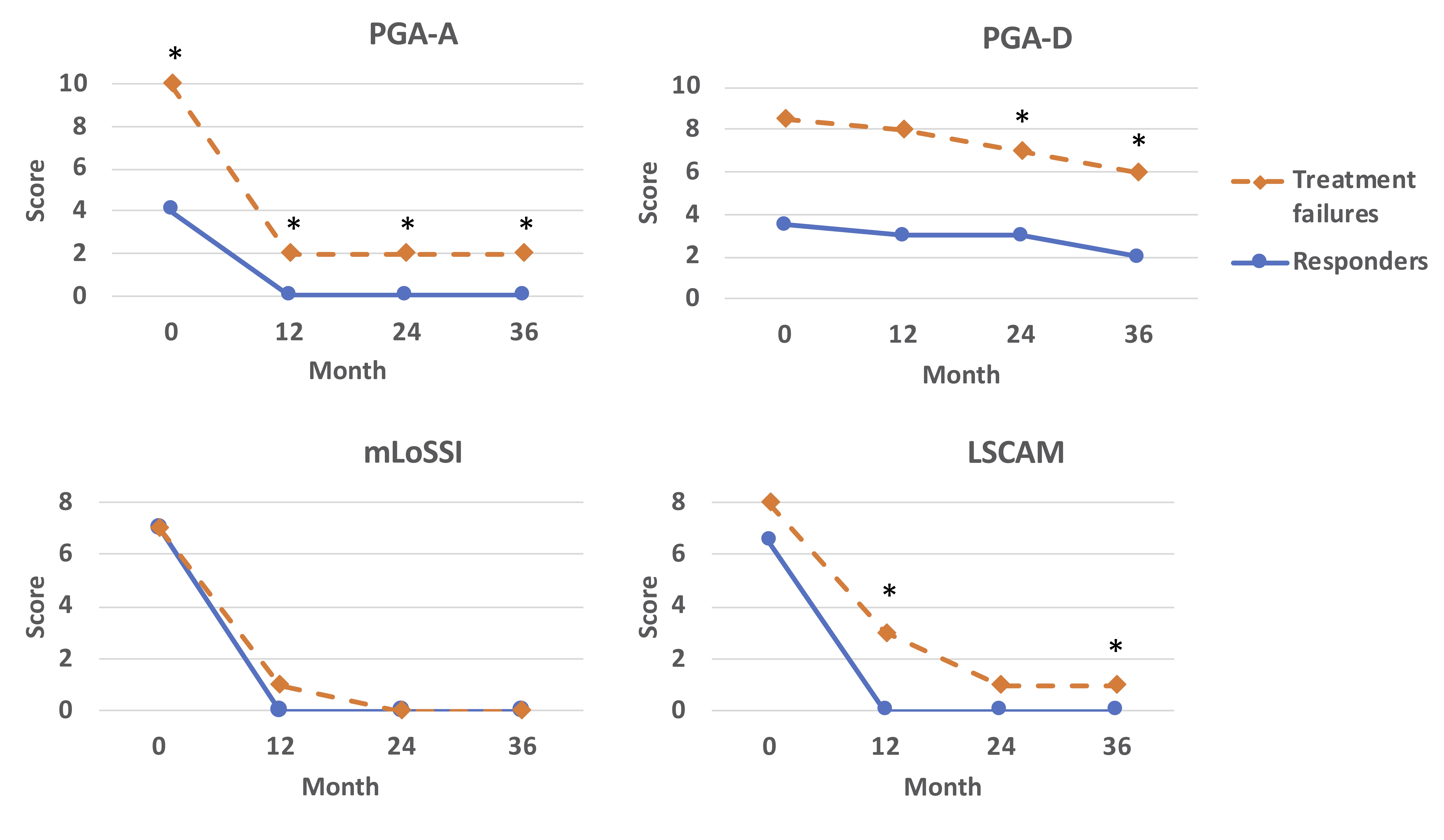
PGA-A and Skin scores declined significantly from 0 to 12 months for both responders and treatment failures. However, patients who experienced TF had higher PGA-A scores than responders at all visits (Figure 2). Skin activity scores, as measured by LSCAM, were also higher in patients who experienced TF at the 12- and 36-month visits than responders; no difference was identified by mLoSSI (Figure 2). PGA-Damage scores were higher at 24 and 36 months in patients who experienced TF than responders.
Conclusion: JLS patients treated with MTX-based standardized regimens were found to achieve further improvement in years 2 and 3 with continued treatment, with 20% able to achieve remission off medicine in year 3. Disease flares occurred in each year and overall, 16 (32%) patients were non-responders, with extracutaneous involvement, especially arthritis, associated with treatment failure. More work is needed to identify the at-risk patients and determine their optimal therapy.
JLS patients in the pilot CTP study were followed after they initiated treatment with one of 3 MTX-based CTPs. The characteristics of all the patients that completed the first year of treatment, patients that ever experienced a treatment failure (treatment failures), and patients that responded to the CTP (responders) are shown. Numbers are shown with % in parentheses unless otherwise stated. The % for number of patients is based upon the total initial cohort (50 patients). The other % are based upon the number of patients in the specific cohort. P-value was calculated for differences between the responders and treatment failures.
Patients initiating treatment with one of 3 MTX-based CTPs (no CS, concomitant oral CS, or concomitant intravenous CS) were followed for up to 36 months. There were 50 patients at 0 months, 44 at 12 months, 39 at 24 months, and 37 at 36 months. Inactive disease was defined as PGA-A = 0, treatment failure as active disease requiring additional treatment besides that specified by CTP.
mLoSSI: modified Localized Scleroderma Skin Severity Index; LSCAM: Localized Scleroderma Cutaneous Activity Measure; PGA-A: Physician global assessment of disease activity; PGA-D: physician global assessment of disease damage.
To cite this abstract in AMA style:
Li S, Thammavongxay A, Ibarra M, Torok K, Ferguson P, Rabinovich C, Fuhlbrigge R, Stewart K, Pope E, Laxer R, Hong S, Mason T, Becker M, Higgins G, Dedeoglu F, CARRA Legacy Registry f. Long-Term Follow-up of Juvenile Localized Scleroderma Patients Treated with Methotrexate-Based Standardized Regimens (Consensus Treatment Plans) [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/long-term-follow-up-of-juvenile-localized-scleroderma-patients-treated-with-methotrexate-based-standardized-regimens-consensus-treatment-plans/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-follow-up-of-juvenile-localized-scleroderma-patients-treated-with-methotrexate-based-standardized-regimens-consensus-treatment-plans/