Session Information
Date: Tuesday, November 14, 2023
Title: (2352–2369) Systemic Sclerosis & Related Disorders – Clinical Poster III: Translational Science
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Acro-osteolysis is a hand complication in systemic sclerosis (SSc) characterized by the destruction of distal digital bone possibly related to repetitive ischemia-reperfusion injury. Diagnosis typically involves a thorough medical history, physical examination, and imaging studies. Unfortunately, there is no reliable serological marker to diagnose SSc acro-osteolysis. The growth factor midkine is expressed during bone formation and fracture repair, and it is upregulated in hypoxia to promote angiogenesis. This study aims to develop a noninvasive and reliable biomarker for acro-osteolysis in patients with SSc.
Methods: This study included SSc registry patients (IRB # 1618579) that had same day capillaroscopy, hand radiographs, and serum collection. Midkine levels were compared between SSc patients with and without acro-osteolysis detected on hand radiographs, and age-matched healthy controls. For all patients, plasma midkine concentrations were determined (MDK ELISA, Invitrogen, USA). An ANOVA test was used to assess differences between plasma midkine levels in SSc patients with acro-osteolysis, without acro-osteolysis and healthy controls.
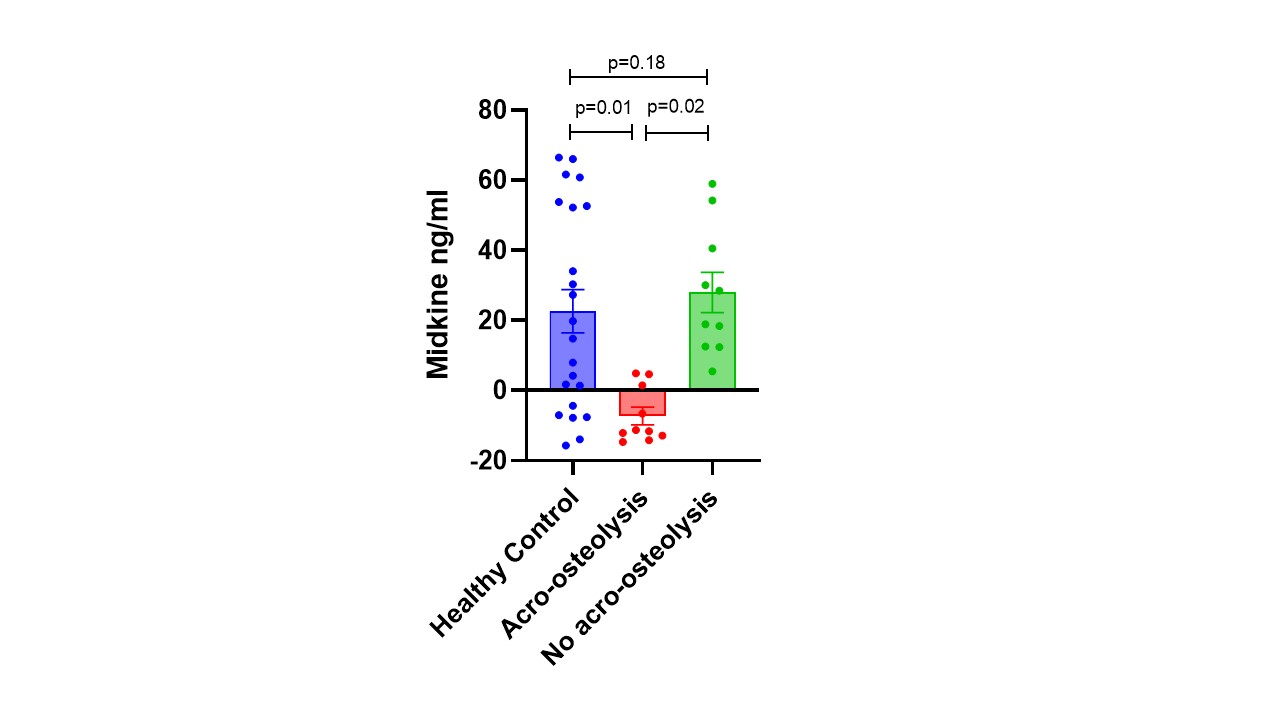
Results: The clinical features of the 20 SSc patients are shown in Table 1. Most patients were white females with an antinuclear antibody (ANA), a SSc-specific autoantibody, limited cutaneous disease, and abnormal nailfold videocapillaroscopy (NVC). There were 10 SSc patients with acro-osteolysis on radiograph and 10 SSc patients without acro-osteolysis. These midkine levels were compared to 20 healthy control samples. The midkine levels were significantly higher in SSc patients with acro-osteolysis compared with no acro-osteolysis (p= 0.02) and healthy controls (p=0.01) (Figure 1). There were no significant differences between healthy controls and SSc patients without acro-osteolysis (p=0.18).
Conclusion: In this small, single-center study, serum level of midkine were significantly reduced in SSc patients with acro-osteolysis when compared to both healthy controls and SSc without acro-osteolysis. Higher levels were associated with intact digital tip on radiograph in SSc patients, which possibly indicates that this growth factor is necessary for maintaining digital health. Ongoing studies are planned to evaluate serial midkine levels as a novel, predictive biomarker for the development of acro-osteolysis in SSc patients.
To cite this abstract in AMA style:
Adeogun G, Gogulamudi V, Balbach M, Donato A, Frech T. Is Midkine a Novel Biomarker for Acro-osteolysis in Systemic Sclerosis? [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/is-midkine-a-novel-biomarker-for-acro-osteolysis-in-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/is-midkine-a-novel-biomarker-for-acro-osteolysis-in-systemic-sclerosis/