Session Information
Date: Tuesday, October 28, 2025
Title: (1990–2014) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster II
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: CSF1 and CSF1R encode a secreted and membrane-bound cytokine and its receptor controlling differentiation of monocytes into macrophages and inflammatory activity of macrophages. There are genetic associations with gout in regulatory regions at each locus which connect with the promoter of each gene in stimulated innate immune cells. The CSF1 locus contains a long noncoding RNA (LINC01768) that is more expressed in stimulated innate immune cells and also a putative enhancer RNA. The CSF1 genetic signal associates with gout but not urate, and CSF1 protein expression is increased in flaring gout patients. We hypothesized that CSF1 has a causal role in gouty inflammation. Our Aim was to gain insights into the molecular control of CSF1 and CSF1R expression.
Methods: MSU crystal (MSUc) was injected into the hindbrain of 2-day larval zebrafish and expression analysis was done by qRT-PCR. For in vitro PBMC studies from 20 gout patients, LPS (1ng/mL), MSUc (300ug/mL), and their combination were used to stimulate cells. Total RNA at 8 hrs was sequenced and reads counted and normalized (to cells-only control). A linear mixed model was fit for CSF1 and CSF1R expression (log2 transformed). Fixed effects were tested for main and two-way interactions of MSUc and LPS. THP-1 cells were stimulated with 30 ng/ml PMA up to 96 hrs to upregulate CSF1 and CSF1R expression. For expression analysis of the putative enhancer RNA and LINC01768, random hexamers were used for reverse transcription and digital PCR was done. CSF1 and CSF1R mRNA levels were quantified using qPCR.
Results: Zebrafish have two orthologs of each of CSF1 and CSF1R (csf1a, csf1b, csf1ra, csf1rb). Injection of MSUc into the hindbrain of zebrafish increased expression of both csf1a and csf1b up to 1.5-fold within 3-9 hrs (Fig 1). However, expression of CSF1R orthologs did not change or decreased. In PBMCs, stimulation with MSUc increased expression of CSF1 with the presence of LPS subduing the increase (Fig 2). In contrast, maximal expression of CSF1R happened under no stimulation, with LPS and MSUc repressing expression. In THP-1 cells PMA treatment induced expression of CSF1, the LINC01768 IPL and the putative enhancer RNA. Maximal expression of the enhancer RNA and LINC01768 preceded that of CSF1. Maximal expression of CSF1R was observed after 48-hours. These CSF1 and CSF1R expression data are consistent with previous research. At CSF1 there were multiple FANTOM5-CAGE multiple transcription start site peaks in monocytes but not in T-cells near the lead SNP (Fig 3). Rs2938616 maps within an H3K27Ac transcriptionally active peak, and there are interactions with both CSF1 and LINC01768. FANTOM6-CAGE data provides evidence for selective expression of the transcription start sites upon PMA stimulation of monocytes. Our data are consistent with a model of genomic control where, in response to stimulation, the gout causal genetic variant mediates induction of the enhancer and LINC01768 RNAs which in turn mediate CSF1 expression.
Conclusion: In diverse experimental systems MSUc induces CSF1 expression, and suppresses CSF1R expression. With the previous genetic association data our findings directly implicate CSF1 in the pathogenesis of gout.
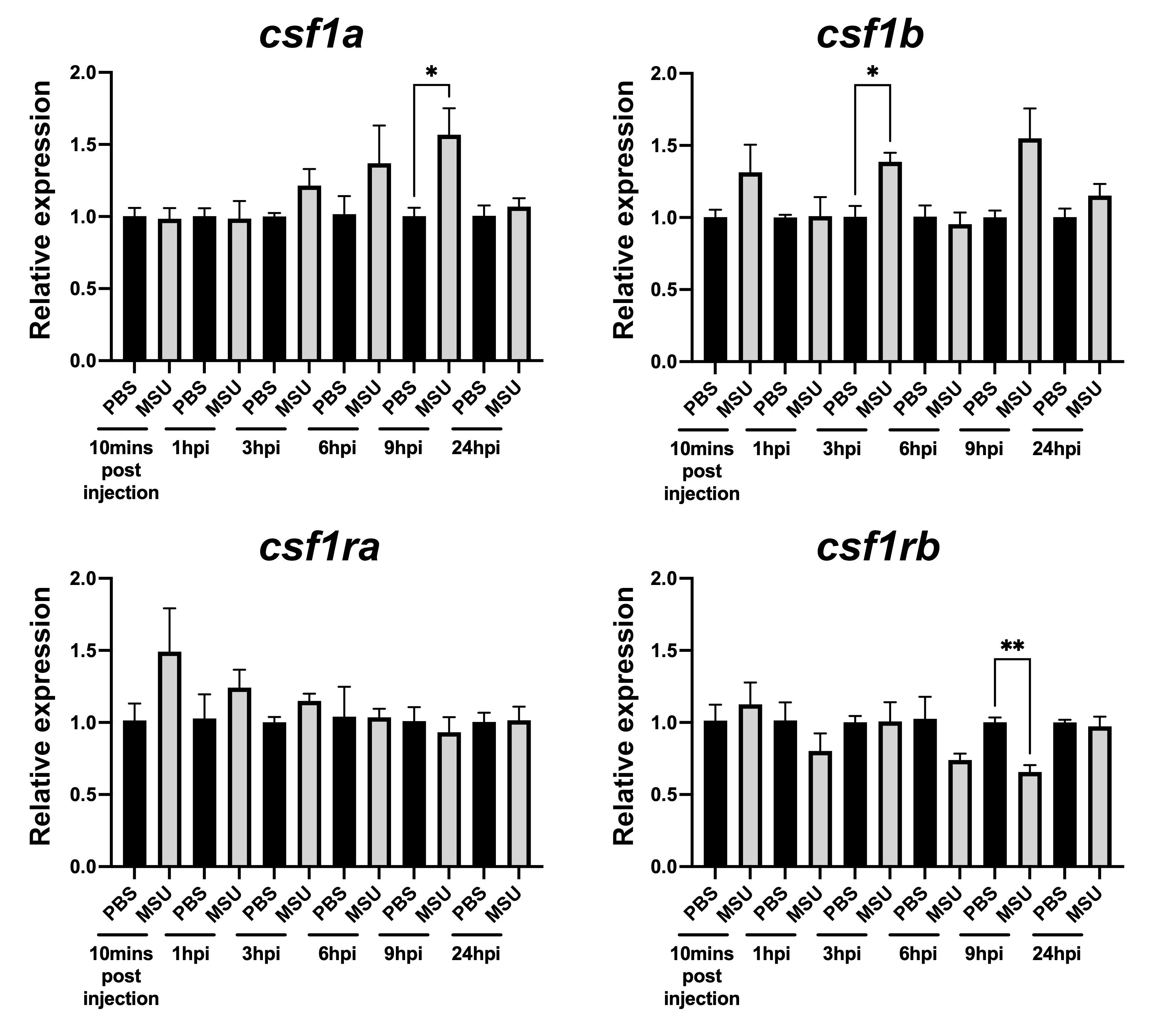 Figure 1. Expression of csf1a, csf1b, csf1ra, csf1rb after injection of MSUc into the hindbrain of 2-day zebrafish larva.
Figure 1. Expression of csf1a, csf1b, csf1ra, csf1rb after injection of MSUc into the hindbrain of 2-day zebrafish larva.
.jpg) Figure 2. Expression of CSF1 and CSF1R in PBMCs in response to MSU crystal, LPS and their combination at 8-hrs.
Figure 2. Expression of CSF1 and CSF1R in PBMCs in response to MSU crystal, LPS and their combination at 8-hrs.
.jpg) Figure 3. Genomic organization of the CSF1 locus (from the UCSC and Zenbu browsers).
Figure 3. Genomic organization of the CSF1 locus (from the UCSC and Zenbu browsers).
To cite this abstract in AMA style:
Merriman T, Hall C, Chien A, Reynolds R, Edberg J, Lertprachakwong P, Darroch H, Leask M, Ozturk M, Kischkel B, Sumpter N, Joosten L, Asmann N. Innate Transcriptional Response and Control of Expression of the Gout-Associated Colony Stimulating Factor-1 (CSF1) and CSF1 Receptor (CSF1R) Genes to Stimuli [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/innate-transcriptional-response-and-control-of-expression-of-the-gout-associated-colony-stimulating-factor-1-csf1-and-csf1-receptor-csf1r-genes-to-stimuli/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/innate-transcriptional-response-and-control-of-expression-of-the-gout-associated-colony-stimulating-factor-1-csf1-and-csf1-receptor-csf1r-genes-to-stimuli/
