Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Juvenile-onset systemic sclerosis (jSSc) is an inflammatory, fibrotic, and vasculopathic disease that causes severe multi-organ dysfunction leading to significant morbidity and early mortality.When patients with adult SSc are refractory to available immunomodulatory therapies clinical studies support the use of autologous stem cell transplant (ASCT), but data is limited in jSSc. Herein, we report the outcomes of five jSSc patients 6 months to 2 years after receiving ASCT.
Methods: All patients were referred to our multidisciplinary pediatric scleroderma center, had disease refractory to ≥3 immunomodulatory therapies, and met criteria for ASCT by severity of lung or skin disease, or both (Table 1). Our ASCT protocol began with stem cell mobilization with cyclophosphamide and filgrastim, collected via pheresis. Conditioning chemoradiation was then administered per an IRB-approved individual treatment plan (Patients 1-3) or per our clinical trial NCT03630211 (Patients 4-5) with rituximab, anti-thymocyte globulin (ATG), total body irradiation 600 cGy with lung and kidney shielding, and cyclophosphamide or thiotepa +/- alemtuzumab. CD34+ selected cryopreserved autologous peripheral blood stem cell graft was infused on day 0. Antimicrobial prophylaxis and viral monitoring (EBV, CMV, adenovirus) followed institutional standards. Corticosteroids were discontinued by week 5 post-ASCT.Clinical outcomes and patient reported measures were collected at baseline (pre-ASCT), 6, 12 and 24 months-post ASCT.
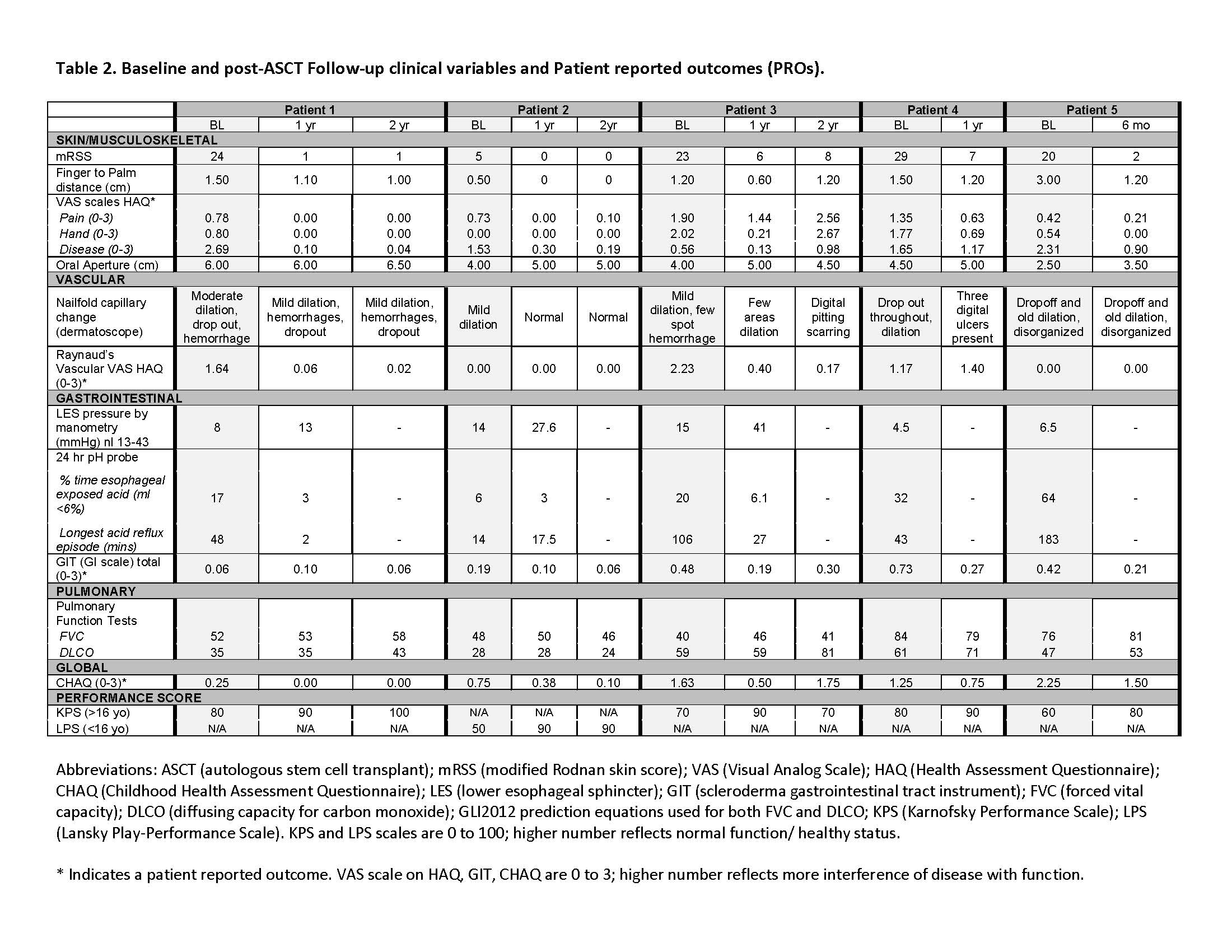
Results: All 5 patients engrafted, with median neutrophil engraftment on day 10.8 (range 10-11) and median platelet engraftment ( >50K) on day 18.8 (range 16-20). Patients globally improved across physician-measured and patient reported outcomes (Table 2).There was an average overall disease HAQ VAS improvement of 75% (range: 48-99%). In the skin/musculoskeletal domain, all 5 patients met the mRSS MCID by their 6-month visit, with an average of 87% improvement (range 74-100%), sustained at 1 and 2 years follow-up. In the gastrointestinal domain, four patients displayed improvement in their GIT by a mean 55% (range 38-68%) and one remained unchanged. Three had repeat lower esophageal manometry with an average improvement of 15 mmHg (range: 5-26 mmHg) associated with an average reduction in esophageal acid exposure by 67%. In the pulmonary domain, FVC overall remained relatively stable, however DLCO improved by >10% among two patients and 5-10% among two other patients. Global function, measured by the CHAQ from a rheumatology standpoint, demonstrated a significant mean reduction among all patients in CHAQ at 6 months by -0.60, surpassing the MCID threefold, and from a BMT standpoint, the performance functional score increased from a mean score of 68 (range: 50-80) to 86 (range: 70-100) toward healthy status.
Conclusion: Among our cohort of refractory jSSc patients with moderate-severe disease, ASCT was a safe and effective intervention that provided sustained global disease modifying improvement throughout 2 years after transplant. Most notable improvements were in skin, gastrointestinal, pulmonary, and patient-reported functional domains.
To cite this abstract in AMA style:
Torok K, Horvei P, Rosser F, Rose-felker K, Sood V, Olsen A, Hogue N, Vandergrift V, Farver L, Mcguire D, Li J, Havrilla H, Alexander J, McIntyre S, Szabolcs P. Improvement in Clinical and Patient-Reported Outcomes for Refractory Juvenile-Onset Systemic Sclerosis (jSSc) 6 Months to 2 Years After Autologous Stem Cell Transplantation (ASCT) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/improvement-in-clinical-and-patient-reported-outcomes-for-refractory-juvenile-onset-systemic-sclerosis-jssc-6-months-to-2-years-after-autologous-stem-cell-transplantation-asct/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improvement-in-clinical-and-patient-reported-outcomes-for-refractory-juvenile-onset-systemic-sclerosis-jssc-6-months-to-2-years-after-autologous-stem-cell-transplantation-asct/