Session Information
Date: Monday, November 9, 2020
Title: RA – Diagnosis, Manifestations, & Outcomes Poster IV: Lifespan of a Disease
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Long-term observational studies on the prediction of favorable outcome (FO) in rheumatoid arthritis (RA) have mostly considered patients baseline characteristics and have rarely evaluated the specific impact of treatments in real world settings.
The objective of this study was to assess the impact of treatments exposure on the 10-year FO in early rheumatoid arthritis (RA).
Methods: The 349 patients of the Etude et Suivi des Polyarthrites Indifférenciées Récentes (ESPOIR) cohort fulfilling ACR/EULAR 2010 criteria at baseline and having complete DAS28 and HAQ data at 10 years were considered in the present study. FO was defined at 10 years as DAS28 ≤ 2.6 and HAQ ≤ 0.5. Three RA treatments were considered: glucocorticoids (GC), conventional synthetic and biologic Disease-Modifying Anti-Rheumatic Drugs (csDMARDs and bDMARDs), which posologies were standardized by the mean of dose quotients (DoseQ). Drug exposure was modelled with Weighted Cumulative Exposure (WCE) variables [1], considering the intensity of drug exposure defined as a weighted function of past doses, and was incorporated into a logistic regression model that also included baseline clinical, biological and radiological characteristics. The predictive performance of this WCE model was compared to models considering on the one hand only baseline characteristics (BSL model) and on the other, baseline characteristics and binary treatments exposure – in other terms, “ever exposed, yes or no” (BIT model).
Results: Overall, FO at 10 years occurred in 158 (45.3%) patients. GC exposure was significantly associated with favorable outcome in univariate analysis only, and therefore was not included in the final WCE combined model.
In the final WCE model, the joint exposure to 1 DoseQ of csDMARD and 1 DoseQ of bDMARD during the 10-year follow-up was not significantly associated with FO compared to patients receiving no treatment: OR=1.10 (95% CI: 0.88-1.38).
Early csDMARD initiation, i.e., as soon as the cohort inclusion, was significantly associated with the occurrence of FO at 10 years (Table 1).
Initiation of a bDMARD between the 3rd month and 2nd year of follow-up in combination with a csDMARD was not significantly better compared to early csDMARD initiation (Table 1).
The final WCE model was better at predicting FO at 10 years compared to the BSL and BIT models: AUC=0.85 (95% CI: 0.81-0.89), AUC=0.65 (95% CI: 0.60-0.71) and AUC=0.72 (95% CI: 0.66-0.79) respectively (Figure 1).
Conclusion: The early initiation of csDMARDs is associated with the occurrence of favorable outcome at 10 years in RA patients. This study confirms that initiating a csDMARD as 1st line treatment and keeping targeted DMARD as 2nd (or more) line is associated with favorable outcome at 10 years, with no loss of chance for the early RA patient.
[1] Dixon WG et al. Immediate and delayed impact of oral glucocorticoid therapy on risk of serious infection in older patients with rheumatoid arthritis: a nested case-control analysis. Ann Rheum Dis 2012;71(7):1128-33.
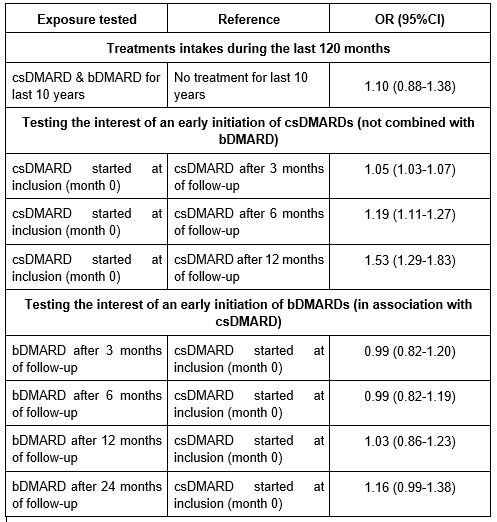 Table 1: odd ratios for the association of patterns of drug regimen with 10-year favorable outcome
Table 1: odd ratios for the association of patterns of drug regimen with 10-year favorable outcome
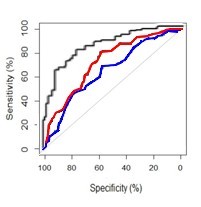 Figure 1: Receiver Operating Characteristic curves of BSL model (blue), BIT model (red) and WCE combined model (black) for 10-year favorable outcome
Figure 1: Receiver Operating Characteristic curves of BSL model (blue), BIT model (red) and WCE combined model (black) for 10-year favorable outcome
To cite this abstract in AMA style:
Kedra J, Hajage D, Lafourcade A, Combe B, Dougados M, Fautrel B. Impact of Treatments on Favorable Outcome over the First 10 Years of Disease in Early Rheumatoid Arthritis: Results from a WCE Model in the ESPOIR Cohort [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/impact-of-treatments-on-favorable-outcome-over-the-first-10-years-of-disease-in-early-rheumatoid-arthritis-results-from-a-wce-model-in-the-espoir-cohort/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-treatments-on-favorable-outcome-over-the-first-10-years-of-disease-in-early-rheumatoid-arthritis-results-from-a-wce-model-in-the-espoir-cohort/
