Session Information
Date: Sunday, November 12, 2023
Title: (0066–0095) T Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Immune checkpoint inhibitor (ICI) therapies used to treat cancer can induce immune related Adverse events (irAEs) such as ICI-induced arthritis (ICI-arthritis). We have previously identified a cytotoxic, CD38hiCD127– CD8 T cell population that is expanded in the joints of ICI-arthritis patients and characterized by an IFN signature. The extent to which these cells can also be found in the circulation and their potential relationship to T cells in inflamed joints has been unclear. In this study we assessed T cell phenotypes in the circulation following ICI therapy and the clonal relationship between anti-PD-1-bound CD8 T cells in the joints and circulation of ICI-arthritis patients.
Methods: We applied mass cytometry on PBMCs from patients with advanced melanoma in the ADAPT-IT trial who received nivolumab plus ipilimumab, with analysis of PBMC pre-treatment and post-2 cycles (n = 48; 31 with paired pre/post samples) . An anti-IgG4 antibody was included to detect nivolumab (an IgG4 antibody) bound on the cell surface. Separately, bulk TCR sequencing was performed on memory CD8 T cells from paired blood and synovial fluid samples of 3 ICI-arthritis patients.
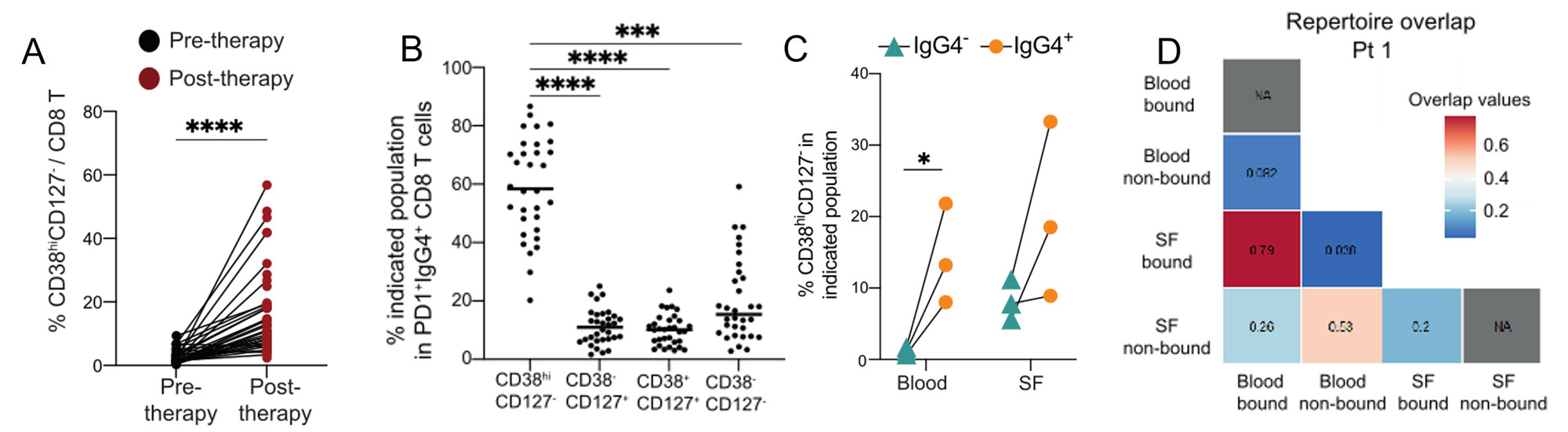
Results: Unbiased clustering and differential abundance analysis of T cell populations in PMBC from before and after treatment identified several changes in T cell populations, including an increase of a GZMB+ CD8 T cells subset (p=0.0002), a FoxP3+CD25+ regulatory CD4 T cell subset (p< 0.0001) and a CCR6+CD161+ Th17 cell subset (p=< 0.0001). The most dramatic change observed across all PBMCs was a significant induction of CD38hiCD127– CD8 and CD4 T cells (p< 0.0001,p< 0.0001) (Fig 1A). Both the CD8 and CD4 CD38hi T cells were often bound by anti-PD-1 therapy and were Ki67+, indicating that they are proliferative (Fig 1B).
We further investigated the anti-PD-1 drug-bound CD8 T cells in paired blood and synovial fluid samples of those with ICI-arthritis. In both blood and synovial fluid, CD38hiCD127– CD8 T cells were more frequent in anti-PD-1-bound samples than non-drug-bound samples (Fig 1C). In ICI-arthritis patients, anti-PD-1 drug-bound CD8 T cells in blood had marked clonal overlap with drug-bound CD8 T cells in synovial fluid (Fig 1D). There was minimal overlap between drug-bound cells in the synovial fluid and non-drug-bound cells in blood.
Conclusion: CD38hiCD127– CD8 T cells are expanded following ICI therapy and exhibit clonal overlap in the synovial fluid and circulation of ICI-arthritis patients. These results suggest that anti-PD-1 therapy directly targets CD8 T cells to induce CD38hi cytotoxic CD8 T cells. Further, drug-bound, activated cells in the circulation may provide a unique view into the activated T cell populations that accumulate within joints of ICI-arthritis patients.
To cite this abstract in AMA style:
Marks K, Singaraju A, Wang R, Adejoorin I, Fein M, Postow M, Chan K, Bass A, Donlin L, Rao D. Immune Checkpoint Inhibitor Therapy Expands an Activated, anti-PD-1 Drug-bound CD8 T Cell Population That Is Clonally Linked in Blood and Synovial Fluid of ICI-arthritis Patients [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/immune-checkpoint-inhibitor-therapy-expands-an-activated-anti-pd-1-drug-bound-cd8-t-cell-population-that-is-clonally-linked-in-blood-and-synovial-fluid-of-ici-arthritis-patients/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immune-checkpoint-inhibitor-therapy-expands-an-activated-anti-pd-1-drug-bound-cd8-t-cell-population-that-is-clonally-linked-in-blood-and-synovial-fluid-of-ici-arthritis-patients/