Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Lupus nephritis (LN) is characterized by considerable variability in its clinical manifestations and histopathological findings. Understanding the cellular and molecular mechanisms underlying this heterogeneity is key for the development of personalized treatments for LN.
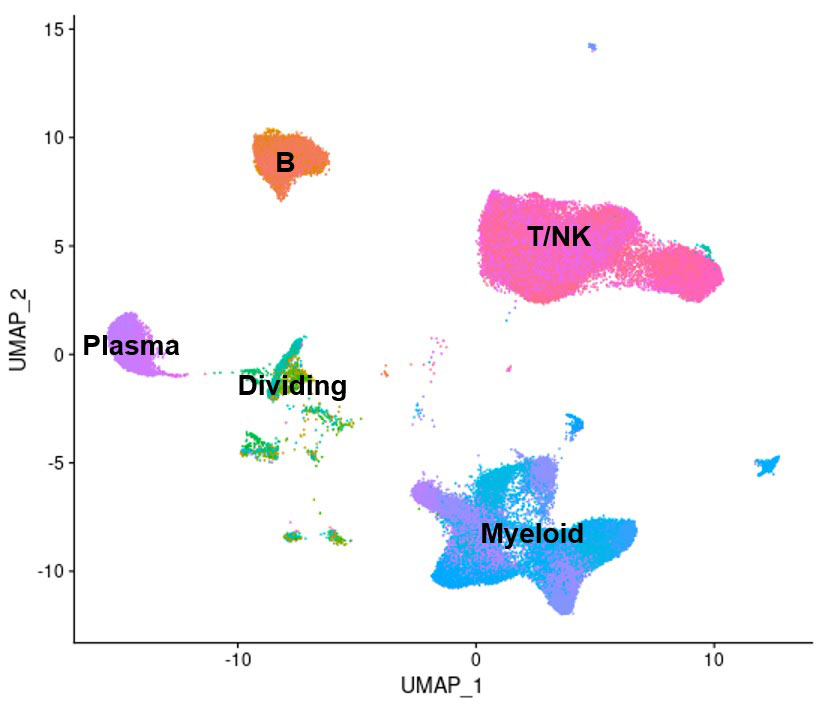
Methods: Droplet-based single-cell RNA-sequencing was applied to the analysis of dissociated kidney samples, collected from 155 LN patients with active kidney disease and 30 living donor controls as part of a large-scale, multi-center study. 73,440 immune cells passing quality control were identified, spanning 134 cell subsets, representing various populations of tissue-resident and infiltrating leukocytes, as well as the activation states these cells assume as part of their disease-related activation and differentiation (Fig. 1). Principal component analysis (PCA) was used to characterize the variability in cell subset frequencies across the LN patients. Relationships between the resulting principal components (PCs) and the demographic, clinical and histopathological features of the patients were then assessed.
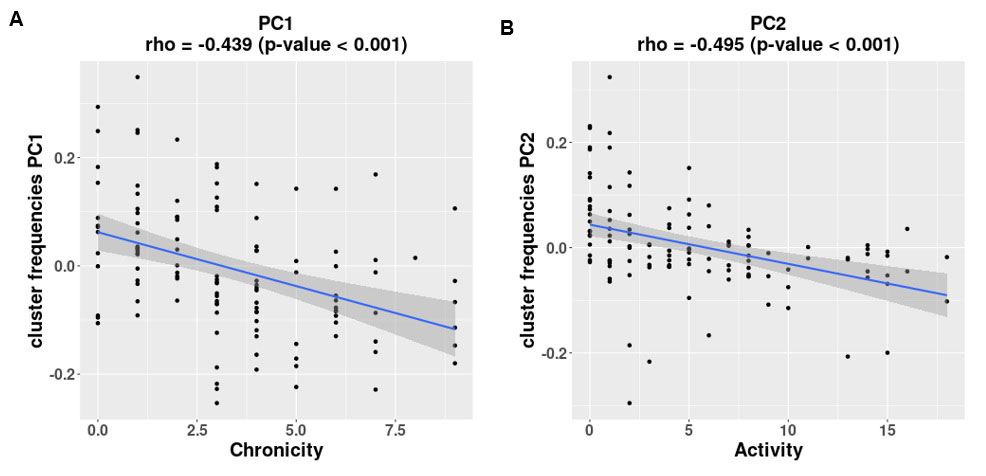
Results: The first PC (PC1), explaining 33% of the total variability in cell subset frequencies, reflected the balance between lymphocytes and monocytes/macrophages. The second PC (PC2), explaining an additional 21% of the total variability, represented the degree of macrophage differentiation to an alternatively activated phagocytic profile. The third and fourth PCs, bringing the total explained variability to 74%, were related to the balance between cell-mediated and humoral immune responses. PC1 was significantly correlated with the Chronicity index, such that patients with a higher percentage of lymphocytes compared to monocytes/macrophages had a higher Chronicity score (rho = -0.439, p-value < 0.001; Fig. 2A). A high degree of macrophage differentiation, as represented by PC2, was associated with a high Activity score (rho = -0.495, p-value < 0.001; Fig. 2B), and, in addition, with proliferative or mixed histology class, compared to pure membranous nephritis (p-value = 0.001, Kruskal–Wallis test). We further identified a significant correlation of these PCs with age; specifically, older patients had a higher relative frequency of lymphocytes compared to monocytes/macrophages, a lesser degree of macrophage differentiation, and a higher representation of cells putatively involved in a humoral immune response compared to a cell-mediated one.
Conclusion: These results identify distinct leukocyte populations active in different LN patients and, possibly, different stages of disease, and suggest potential therapeutic targets, that must be validated in mechanistic studies. This approach may pave the way to personalized treatment of LN.
To cite this abstract in AMA style:
Arazi A, Mears J, Eisenhaure T, Xiao Q, Hoover P, Rao D, Berthier C, Fava A, Gurajala S, Peters M, Jones T, Apruzzese W, Barnas J, Furie R, Davidson A, Hildeman D, James J, Guthridge J, Dall'Era M, Wofsy D, Izmirly P, Belmont H, Clancy R, Kamen D, Putterman C, Tuschl T, McMahon M, Grossman J, Kalunian K, Weisman M, Kretzler M, Brenner M, Anolik J, Petri M, Buyon J, Raychaudhuri S, Hacohen N, Diamond B, RA/SLE Network t. Immune Cell Heterogeneity in Lupus Nephritis Kidneys and Its Relation to Histopathological Features [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/immune-cell-heterogeneity-in-lupus-nephritis-kidneys-and-its-relation-to-histopathological-features/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immune-cell-heterogeneity-in-lupus-nephritis-kidneys-and-its-relation-to-histopathological-features/