Session Information
Date: Monday, November 13, 2023
Title: (0934–0964) Systemic Sclerosis & Related Disorders – Basic Science Poster
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Systemic sclerosis (SS), also known as scleroderma, is a chronic autoimmune disease caused by inflammation, tissue fibrosis, and vasculopathy. Although the pathological mechanisms of SS are unclear, proinflammatory cytokines, such as IL-4 and IL-17, and the tissue microenvironment are important factors in its development. In this study, we aim to investigate whether T cells induce fibrosis in SS and assess the effect of simultaneous regulation of T cells and fibroblasts via dedicated signals.
Methods: SKG mice, T cell-mediated autoimmune murine models originated from a spontaneous point mutation in ZAP70, and BALB/c mice (control) were subcutaneously injected with bleomycin (BLM) to induce SS-like phenotypes including fibrosis. The degree of fibrosis, and expression levels of inflammatory or profibrotic cytokines were determined by histological assessments using immunohistochemistry and confocal microscopy. Potential molecular mechanisms related to T cell activation were determined in the same mice both in vitro and ex vivo. The profibrotic effects of human T cells were evaluated using a humanized murine SS model induced by peripheral blood mononuclear cells (PBMCs) from SS patients.
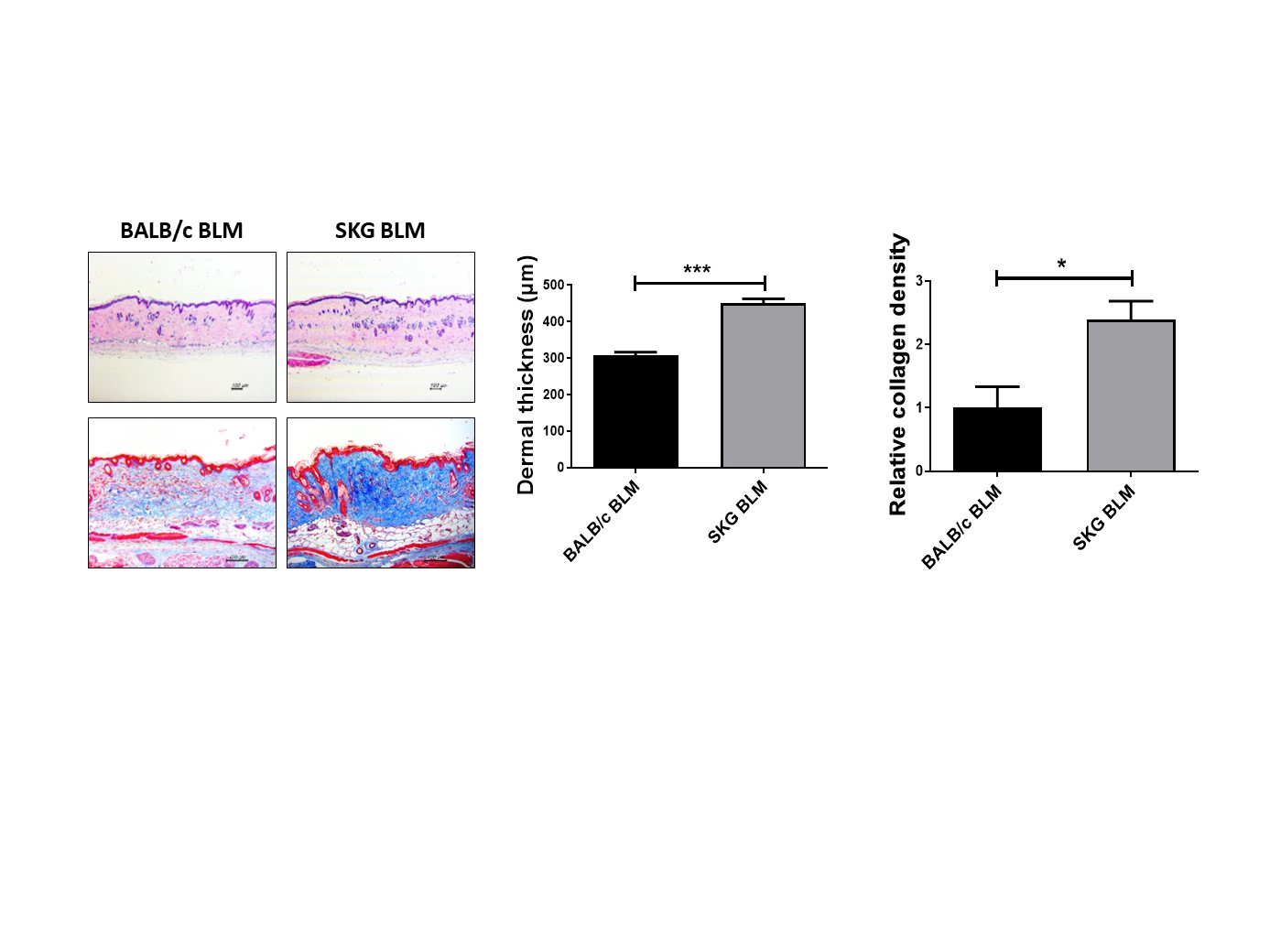
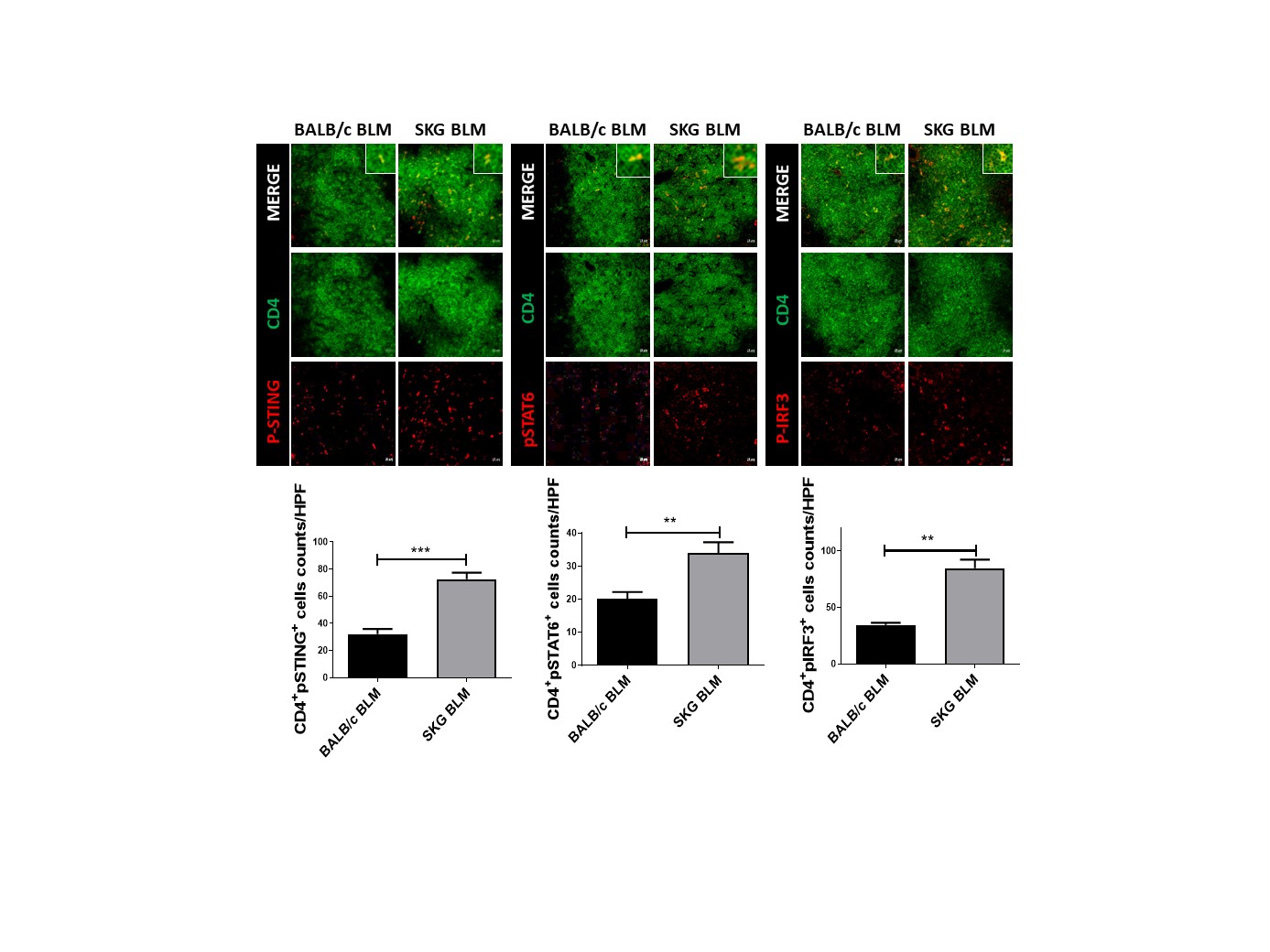
Results: Dermal thickness and expression of fibrosis markers in skin tissue were increased in BLM-treated SKG mice compared to control mice. Infiltration of T cells, particularly Th2 and Th17 cells and expression of related cytokines were increased in skin tissues from BLM-treated SKG mice than those from controls. In CD4+ T cells from SKG mice, the STING pathway including downstream factors such as STAT6 and IRF3 was activated, subsequently resulting in increased populations of IL-4 and IFN-α-producing CD4+ T cells. IL-4 and IFN-α directly promoted fibrosis in skin fibroblasts via the STING pathway. Increased fibrosis and infiltration of IL-4 and IFN-α-producing CD4+ T cells were replicated in humanized mice induced by PBMCs from SS patients. Lastly, pharmacological inhibitions of STING as well as STAT6 reduced fibrosis in the murine models.
Conclusion: Our findings suggest that STING-induced production of IL-4- and IFN-α by CD4+ T cells is a key factor of pathological fibrosis in SS. The STING and STAT6 signaling pathways can be potential therapeutic targets in SS.
To cite this abstract in AMA style:
Yang J, Lee K, Woo J, Jeong H, Choi J, Kwon E, Cho M, Park S. IL-4+ and IFN-α+ Profibrotic T Cells Aggravate Systemic Sclerosis via STIM1/STING Signaling in SKG Mice [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/il-4-and-ifn-%ce%b1-profibrotic-t-cells-aggravate-systemic-sclerosis-via-stim1-sting-signaling-in-skg-mice/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/il-4-and-ifn-%ce%b1-profibrotic-t-cells-aggravate-systemic-sclerosis-via-stim1-sting-signaling-in-skg-mice/