Session Information
Date: Monday, October 27, 2025
Title: (0978–1006) T Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Interleukin-23 (IL-23) is a key cytokine in the pathogenesis of psoriatic diseases, as demonstrated by the clinical success of IL-23-targeted therapies. Monocytes are a primary source of IL-23, contributing to the inflammatory milieu.IL-23 is known to drive the pathogenicity of T-helper (Th) cells with a CCR6⁺ phenotype; however, its specific effects on distinct memory T cell subsets remain poorly defined. The CCR6⁺ Th population is heterogeneous, comprising Th17 cells, which predominantly produce IL-17A, and Th17.1 cells, which mainly secrete interferon-gamma (IFN-γ). We aim to characterize the interactions between IL-23 and the different CCR6+ Ths, which is essential for advancing our understanding of psoriatic disease mechanisms and improving therapeutic strategies.
Methods: Human CCR6+, CCR6-, Th17, Th17.1 and classical Th1 cells were sorted from peripheral blood mononuclear cells (PBMCs). Transcription of IL-23R was determined with RT-PCR. CCR6+, CCR6-, Th17, Th17.1 and Th1 cells were stimulated with αCD3/αCD28, in the presence or absence of IL-23 for 72h before measuring cytokine secretion with ELISA. CD14+ monocytes were isolated using MACS and cocultured with Th cell populations before measuring IFN-γ secretion with ELISA.
Results: Ex vivo analysis revealed that CCR6⁺ T-helper cells expressed significantly higher levels of IL-23R mRNA compared to CCR6⁻ counterparts. Upon stimulation with IL-23, CCR6⁺ Th cells secreted significantly more interferon-gamma (IFN-γ), but not interleukin-17A (IL-17A). To account for the heterogeneity within the CCR6⁺ population, memory Th17, Th17.1, and classical CCR6⁻ Th1 cells were further sorted and analyzed. Both Th17 and Th17.1 cells exhibited higher IL-23R expression than Th1 cells. Following IL-23 stimulation, Th17.1 cells produced significantly more IFN-γ, whereas Th17 and Th1 cells did not. IFN-γ was found to induce IL-23 production in CD14⁺ monocytes. In co-culture experiments with monocytes, Th17.1 cells secreted significantly more IFN-γ than classical Th1 cells.
Conclusion: T-helper cell subsets exhibit distinct responses to IL-23, particularly in their ability to produce IFN-γ. Our data show that IL-23 selectively induces IFN-γ secretion in Th17.1 cells, but not in Th17 or classical Th1 cells. Additionally, IFN-γ promotes IL-23 production by monocytes, suggesting a proinflammatory feedback loop that may amplify Th17.1-mediated inflammation. When co-cultured with monocytes, Th17.1 cells produced more IFN-γ than Th1 cells, further underscoring their heightened pro-inflammatory potential. These findings highlight a unique IL-23–IFN-γ axis in Th17.1 cells that may contribute significantly to local tissue inflammation in psoriatic diseases.
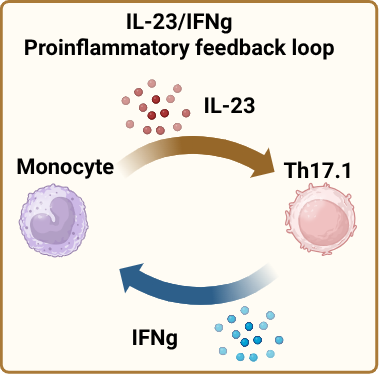 Schematic overview of the IL-23/IFNg proinflammatory feedback loop.
Schematic overview of the IL-23/IFNg proinflammatory feedback loop.
To cite this abstract in AMA style:
van Heeswijk B, Razawy W, Mus-Otten A, Asmawidjaja P, Leenen P, Lubberts E. IL-23 upregulates IFN-γ secretion in Th17.1, but not in Th17 or classical Th1 cells [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/il-23-upregulates-ifn-%ce%b3-secretion-in-th17-1-but-not-in-th17-or-classical-th1-cells/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/il-23-upregulates-ifn-%ce%b3-secretion-in-th17-1-but-not-in-th17-or-classical-th1-cells/
