Session Information
Date: Saturday, November 12, 2022
Title: RA – Animal Models Poster
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: We have previously reported that IL-18 levels in the lungs, serum and bronchoalveolar lavage fluid (BALF) were increased in patients with idiopathic pulmonary lung fibrosis (Am J Respir Cell Mol Biol. 2004;31:619-625). Furthermore, we have demonstrated critical roles of IL-18 in the mouse acute lung injury which is resulting in death from interstitial pneumonia induced by administration of IL-18 plus IL-2 and bleomycin-induced lung fibrosis model (Blood. 2002;99:1289-9, Am J Respir Cell Mol Biol. 2009;41:661-70). Reduced neutrophil accumulation in the lung tissues obtained from bleomycin-treated IL-18 receptor-α (IL-18R α)- and IL-18-deficient mice was observed (Blood. 2002; 99:1289-9). We have also reported that IL-18 receptor complex could be serum biomarker of RA and these evidence clearly indicate the involvement of IL-18 and its receptor system in inflammatory arthritis (Arthritis Res Ther. 2011;13:R52). However, it remains unclear how the IL-18/IL-18Rα signaling pathway functions in the autoimmune inflammatory arthritis. This study investigated the contribution of IL-18/IL-18Rα signaling in autoantibody-induced arthritis.
Methods: C57BL/6 IL-18Rα gene deficient (-/-) mice were used in K/BxN serum transfer arthritis. Synovial tissue resident neutrophils were identified myeloperoxidase-positive cells by immunohistochemistry. Murine fibroblast-like synoviocytes (FLS) were stimulated with recombinant mouse IL-1β or IL-18. mRNA levels of IL-18, CXCL1, IL-8 and IL-1β were analyzed in cultured cells. Synovial tissue expression of IL-18, IL-1β and CXCL1 mRNA was also investigated by RT-PCR.
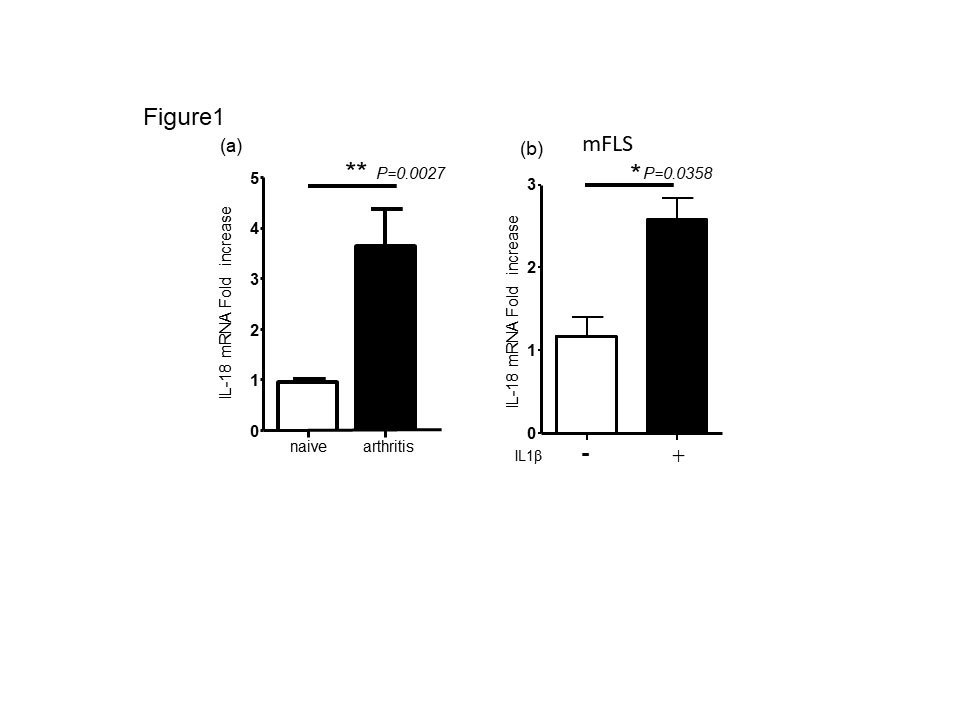
Results: IL-18 mRNA expression exhibited significant upregulation in mouse inflamed synovial tissue during K/BxN serum transfer arthritis (Fig1a). Consistent with in vivo data, mouse FLS can produce IL-18 mRNA after stimulation with recombinant IL-1β (Fig 1b).
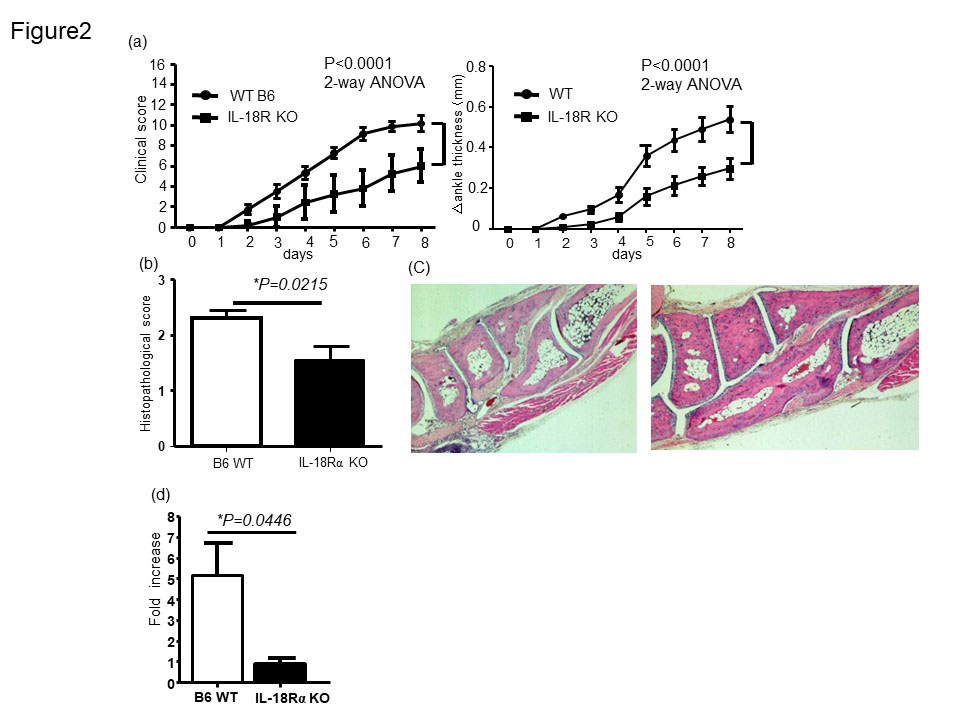
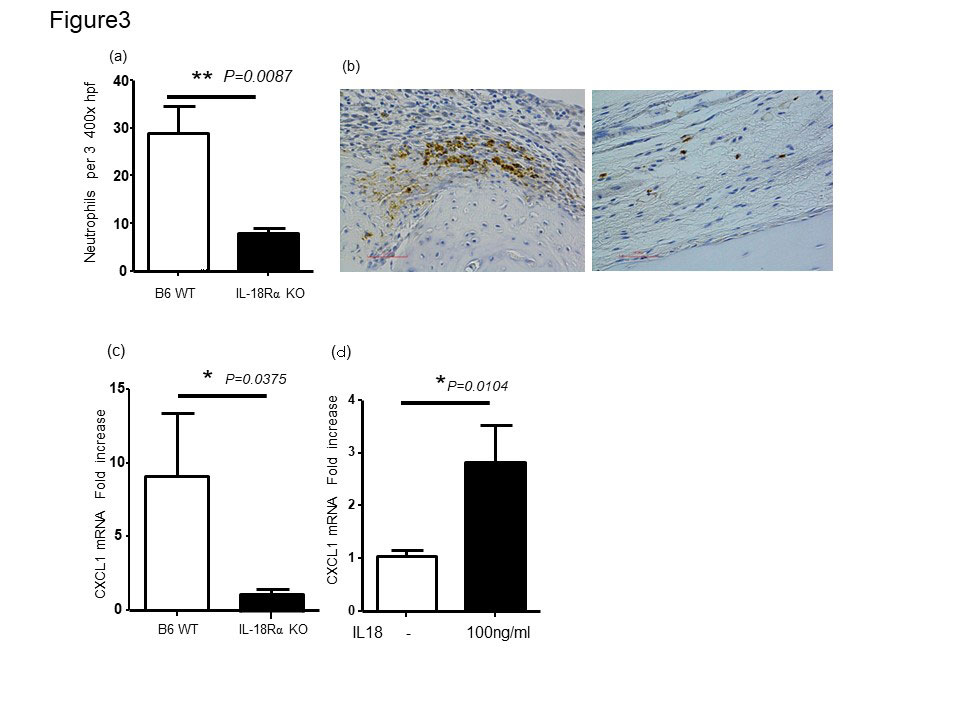
In IL-18Rα-/- mouse, we observed a less disease severity that was accompanied by lower IL-1β gene expression in the joints as compared to WT control mice (Fig2a and d). Histomorphometric quantification of the arthritic changes in the joint tissues confirmed the clinical assessment, with significant decreases in histopathological scores in IL-18Rα−/− (Fig. 2b and c). Infiltration of neutrophils in the arthritic synovium was significantly reduced in IL-18Rα−/−mice compared to that of WT B6 mice. IL-18 has been reported to induce CXC chemokines by synovial fibroblasts in rheumatoid arthritis (Figure3 a and b). We examined the expression of neutrophil chemotaxis CXCL1 in the mouse ankle tissues and was significantly reduced in the arthritic mouse ankles obtained from IL-18 Rα−/− mice (Fig3c). Stimulation of mouse FLS in vitro with recombinant mouse IL-18 resulted in increased expression of CXCL1 (Figure3d). These results suggested the potential contribution of IL-18 to neutrophil recruitment via inducing chemokine CXCL1 from murine FLS during arthritis.
Conclusion: IL-18/IL-18Rα signaling contributes to autoantibody-induced arthritis via inducing neutrophil recruitment. Inhibition of IL-18/IL-18Rα signaling could be an attractive therapeutic potential target in RA.
To cite this abstract in AMA style:
Kaieda S, Hoshino T. IL-18 Receptor-α Signaling Pathway Contributes to Autoantibody-induced Arthritis via Neutrophil Recruitment [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/il-18-receptor-%ce%b1-signaling-pathway-contributes-to-autoantibody-induced-arthritis-via-neutrophil-recruitment/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/il-18-receptor-%ce%b1-signaling-pathway-contributes-to-autoantibody-induced-arthritis-via-neutrophil-recruitment/