Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Previous studies have demonstrated clinical, epidemiologic, histopathologic, and genetic overlap between advanced rheumatoid arthritis-associated interstitial lung disease (RA-ILD) and Idiopathic Pulmonary Fibrosis (IPF). Corresponding work from our group has shown that a number of serum protein biomarkers of IPF are shared with RA-ILD. We therefore assessed the expression of IPF-related proteins in different stages of RA-ILD to support the pathophysiologic overlap between these disorders and to develop candidate markers of disease progression.
Methods: We used multiplex ELISA to assess serum levels of selected cytokines, chemokines, and remodeling proteins in an established cohort of IPF patients enrolled through the University of Pittsburgh Simmons Center for ILD. Comparison of serum molecular profiles between IPF patients and healthy controls identified markers associated with IPF that were then assessed in two independent cohorts of RA patients (VA, non-VA) with different stages of clinically and radiographically defined ILD. Serum concentrations of designated proteins were normalized based on cohort-specific means/standard deviations (Normalized Value=(serum concentration-cohort mean)/cohort SD). A composite score was then calculated for each individual by adding normalized values for designated markers. Mann U Whitney testing was used to compare composite scores in individuals classified as RA-no ILD, RA-subclinical ILD (HRCT abnormalities, no symptoms), and RA-clinically evident ILD.
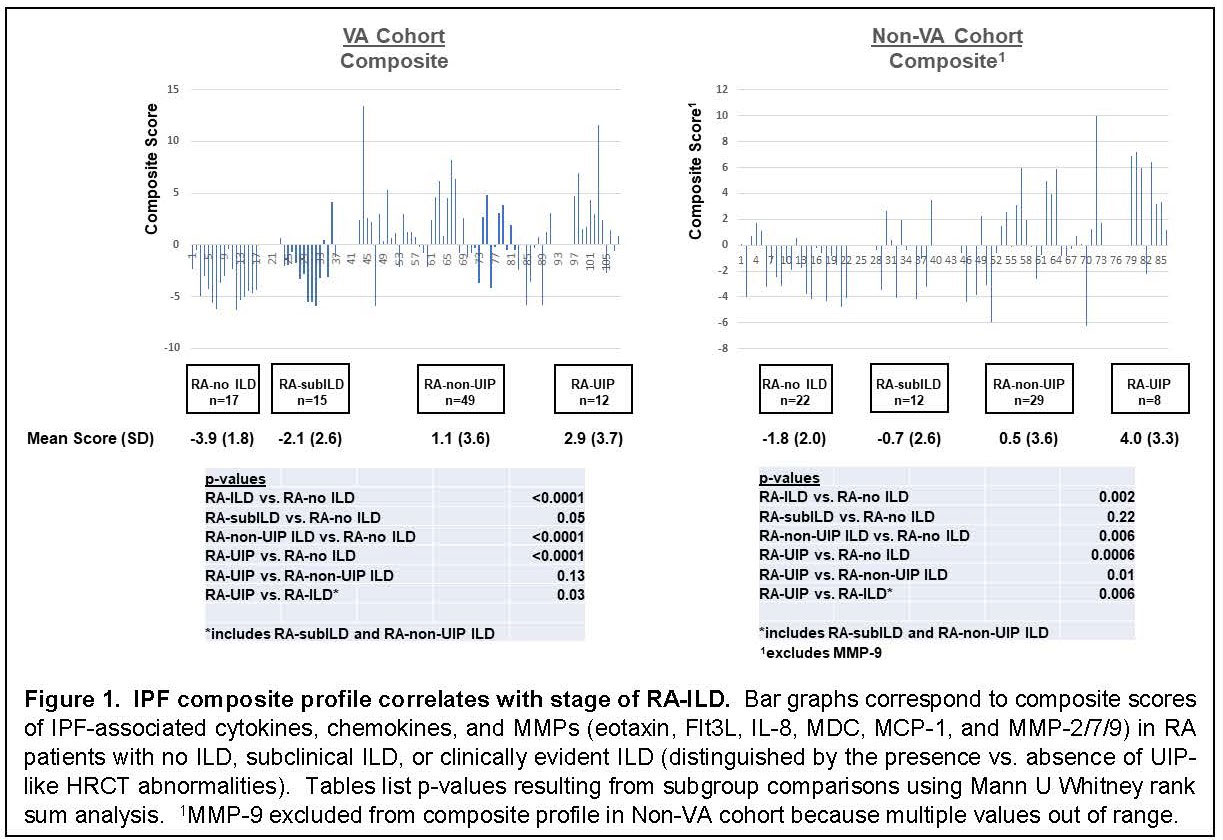
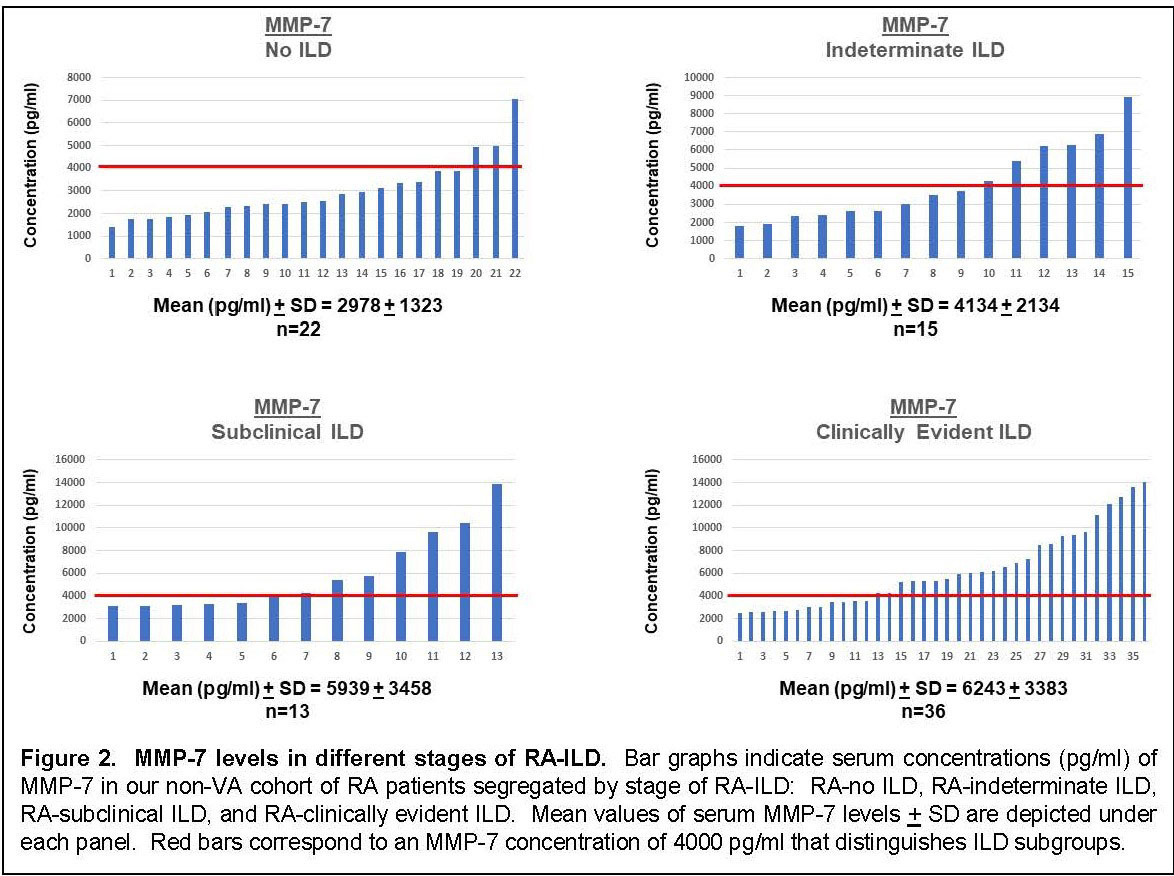
Results: Multiplex ELISA comparison of IPF (n=100) and healthy controls (n=38) revealed that eotaxin, Flt3L, IL-8, MDC, MCP-1, and MMP-2/7/9 were significantly associated with IPF after adjustment for age, sex, and smoking history. Assessment of serum concentrations of these proteins in our VA (n=93) and non-VA (n=71) cohorts of RA/RA-ILD revealed statistically significant associations between composite profiles of these IPF markers and RA-ILD that increased proportionally with stage/severity of disease (Fig 1). Despite clear associations between this IPF molecular signature and clinically evident RA-ILD (UIP and non-UIP) in both the VA and non-VA cohorts, several individuals with subclinical RA-ILD also had elevated composite scores. More detailed analysis of MMP-7 levels in extended subsets (also encompassing radiographically indeterminate ILD) of our non-VA cohort revealed a similar dose response based on stage/severity of RA-ILD—and again demonstrated elevated levels in a subgroup of patients with early stage disease (Fig 2).
Conclusion: IPF-associated molecular profiles are highly correlated with RA-ILD, particularly in UIP and non-UIP subsets of clinically evident RA-ILD. Strikingly, these associations emerge in both VA and non-VA cohorts of RA-ILD, despite significant demographic differences and variable associations (not shown) with individual proteins in this profile. More importantly, the gradient of IPF-associated mediator levels in early RA-ILD supports a potential pathogenic connection with more advanced disease and suggests that this molecular signature may identify individuals with early stages of RA-ILD who are at increased risk of disease progression.
To cite this abstract in AMA style:
Kass D, Nouraie M, Zhang Y, England B, Mikuls T, Kerr G, Reimold A, Gibson K, Glassberg M, Dellaripa P, Doyle T, Oddis C, Ascherman D. Idiopathic Pulmonary Fibrosis Molecular Profile Correlates with Stage of Rheumatoid Arthritis-Associated Interstitial Lung Disease [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/idiopathic-pulmonary-fibrosis-molecular-profile-correlates-with-stage-of-rheumatoid-arthritis-associated-interstitial-lung-disease/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/idiopathic-pulmonary-fibrosis-molecular-profile-correlates-with-stage-of-rheumatoid-arthritis-associated-interstitial-lung-disease/