Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with rheumatoid arthritis (RA) are at an increased risk for comorbid chronic lung disease, with premature mortality. Therapies for RA-associated lung disease are limited. Organic dust extract (ODE)-induced airway inflammation model was previously combined with the collagen-induced arthritis (CIA) model to model RA-related lung disease. The combination of CIA+ODE resulted in increased arthritis severity and higher levels of anti-malondialdehyde-acetaldehyde (MAA) and anti-cyclic citrullinated (CIT) protein antibodies (vs. CIA or ODE alone). Co-exposure also promoted pro-fibrotic lung inflammatory features. CCR2+ monocytes migrate to lung tissues under inflammatory conditions to influence inflammatory fibrotic consequences and may be a target for therapies. We aimed to determine whether CCR2+ monocytes were recruited to the lungs in this mouse RA-lung disease model, and whether anti-inflammatory omega-3 fatty acid derivatives (DHA, resolvin-D1, and maresin-1) would reduce inflammatory responses in vitro from monocyte stimulation with ODE and/or autoreactive antigens.
Methods: Arthritis prone DBA/1J mice were assigned to 1 of 4 groups: Sham (saline injection/saline inhalation), CIA (CIA injection/saline inhalation), ODE (ODE inhalation/saline injection), or CIA+ODE treatment for 5 weeks as previously established. CCR2 expression was quantified using confocal microscopy in murine lung sections. In cell culture studies, human THP-1 monocytes were exposed to varying doses of: ODE, CIT peptides, and MAA-adducted protein, and the combination of ODE + autoantigens. In parallel experiments, cells were pre-treated for one hour with docosahexaenoic acid (DHA) or its metabolites (maresin-1 and resolvin-D1) before stimulation. TNF-α was quantitated in cell-free culture supernatant by ELISA.
Results: In animal studies, CCR2 expression was significantly increased in lung tissues of treated animals CIA+ODE >CIA >ODE vs. sham (Figure 1; N=5 mice/group). In monocyte studies, TNF-α levels were significantly increased following stimulation with ODE (mean ± SEM pg/ml; 1104.2 ± 91.7), MAA (167.8 ± 19.3), and CIT (75.7 ± 22.4) as compared to control (7.4 ± 4.0) (N=9; p< 0.05). No additive effects were demonstrated when agents were combined. Pretreatment with resolvin-D1 significantly reduced ODE-induced TNF-α at 4 hours (75% reduction) and reduced MAA (58%) and CIT (55%) stimulated TNF-α release at only 48 hours. Pretreatment with DHA or maresin-1 did not reduce ODE or autoantigen stimulated TNF-α release. No cytotoxicity was detected with respective treatments.
Conclusion: Arthritis induction promotes the recruitment of CCR2+ monocytes to the lung and this response is augmented by airborne biohazard exposures to suggest that targeting recruited inflammatory monocytes may be a potential strategy. Reduction in TNF-α levels with resolvin-D1 pretreatment in monocytes strengthens the need of future in vivo studies to define whether specific dietary interventions focused on the omega-3 fatty acid resolvin-D1 might help in the prevention and/or treatment of RA-related lung disease.
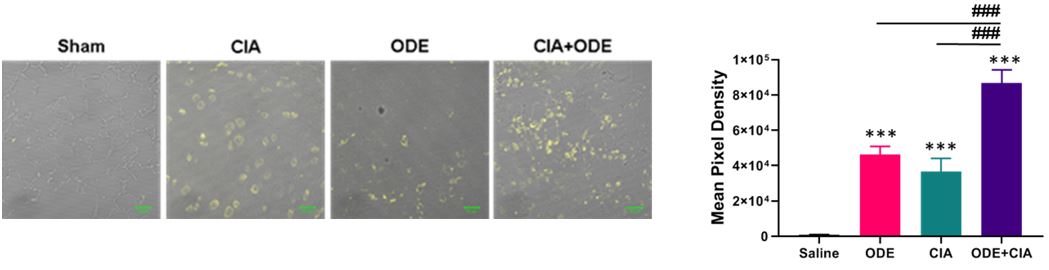 Figure 1. CCR2+ monocytes are increased with ODE and CIA treatment conditions in murine lungs. Representative image (1 of 5) of CCR2 expression (yellow) of lung tissue from each treatment group. Bar graph depicts mean with standard error bars of CCR2 staining (N=5). Statistical difference ***p < 0.001 vs. saline/sham control and ###p < 0.001 denoted by line between groups.
Figure 1. CCR2+ monocytes are increased with ODE and CIA treatment conditions in murine lungs. Representative image (1 of 5) of CCR2 expression (yellow) of lung tissue from each treatment group. Bar graph depicts mean with standard error bars of CCR2 staining (N=5). Statistical difference ***p < 0.001 vs. saline/sham control and ###p < 0.001 denoted by line between groups.
To cite this abstract in AMA style:
Barry A, Thiele G, Mikuls T, Duryee M, Nelson A, Gaurav R, England B, Poole J. Identification of Recruited CCR2+ Inflammatory Monocytes in a Mouse Model of RA-associated Lung Disease with Potential Role for resolvin-D1 in Reducing Monocyte Inflammatory Responses [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/identification-of-recruited-ccr2-inflammatory-monocytes-in-a-mouse-model-of-ra-associated-lung-disease-with-potential-role-for-resolvin-d1-in-reducing-monocyte-inflammatory-responses/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/identification-of-recruited-ccr2-inflammatory-monocytes-in-a-mouse-model-of-ra-associated-lung-disease-with-potential-role-for-resolvin-d1-in-reducing-monocyte-inflammatory-responses/
