Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Mitochondria are organelles that possess several bacterial features such as a double-stranded genome with hypomethylated CpG islets, formylated proteins, and a double membrane composed of cardiolipin. In systemic lupus erythematosus (SLE), mitochondria and their inner components are released into the extracellular space, potentially eliciting a pro-inflammatory response by the immune system. While cardiolipin and mitochondrial DNA (mtDNA) and RNA (mtRNA) are confirmed targets of autoantibodies, antigenic mitochondrial proteins in SLE remain to be identified. Herein, we aim to characterize the antigenic protein repertoire recognized by anti‑mitochondrial antibodies in SLE patients.
Methods: SLE (n=87) and healthy controls (n=30) agreed to participate to our systemic autoimmune rheumatic diseases biobank and database. Sociodemographic and disease characteristic variables [e.g., ACR classification criteria, SLEDAI-2K, SLICC/ACR damage index (SDI), comorbidities] as well as serum samples were collected at the time of the inclusion of the patients in the systemic autoimmune rheumatic disease biobank and data repository. The anti-dsDNA, IgGs against anticardiolipin or anti-β2GPI (i.e., cut-offs of 40 UPL or above the 99th percentile of controls) were measured by ELISA. Lupus anticoagulant assay was performed, following international guidelines for this functional assay. Healthy controls were recruited under the conditions of having no known illnesses and infectious symptoms at the time of the blood draw. Autoantibodies against whole mitochondria (AwMA), mtDNA (AmtDNA) and mtRNA (AmtRNA) were previously assessed. Sera of either 10 healthy donors or 10 SLE patients with high titers of AwMA were pooled and incubated with sources of mitochondrial antigens (Figure 1) and IgG were isolated, using Dynabeads-protein G. Identification of mitochondrial proteins associated to IgGs were performed in triplicates by shotgun proteomic analysis. Immunoreactivities to C1qBP and Mfn1 were assessed by direct ELISA. Comparisons between groups were performed using the Wilcoxon-Mann-Whitney test. Spearman correlations were calculated to see associations between AMA and antibodies assessed in clinical laboratory.
Results: We identified 1345 proteins, 431 of which were associated with the mitochondrial proteome. Autoantibodies to two candidates, namely to the complement component 1 Q subcomponent-binding protein (C1qBP) and mitofusin 1 (Mfn1) were significantly increased in SLE patients compared to healthy individuals (anti-C1qBP: p=0.0167, anti-Mfn1: p=0.0022. Figure 2). Patients with positive lupus anticoagulant had increased anti-C1qBP (p=0.049), while anti-Mfn1 levels were increased in patients positive for antiphospholipids (p=0.011). Levels of Anti-Mfn1 were associated with those of IgGs to anticardiolipin (rs=0.454; p< 0.0001), anti-β2GPI (rs=0.261; p=0.019) and anti-dsDNA (rs=0.443; p=0.039) (Table 1).
Conclusion: These results suggest that autoantibodies to C1qBP and Mfn1 are comprised represented within the AMA repertoire in SLE and display different associations with serological criteria in the disease.
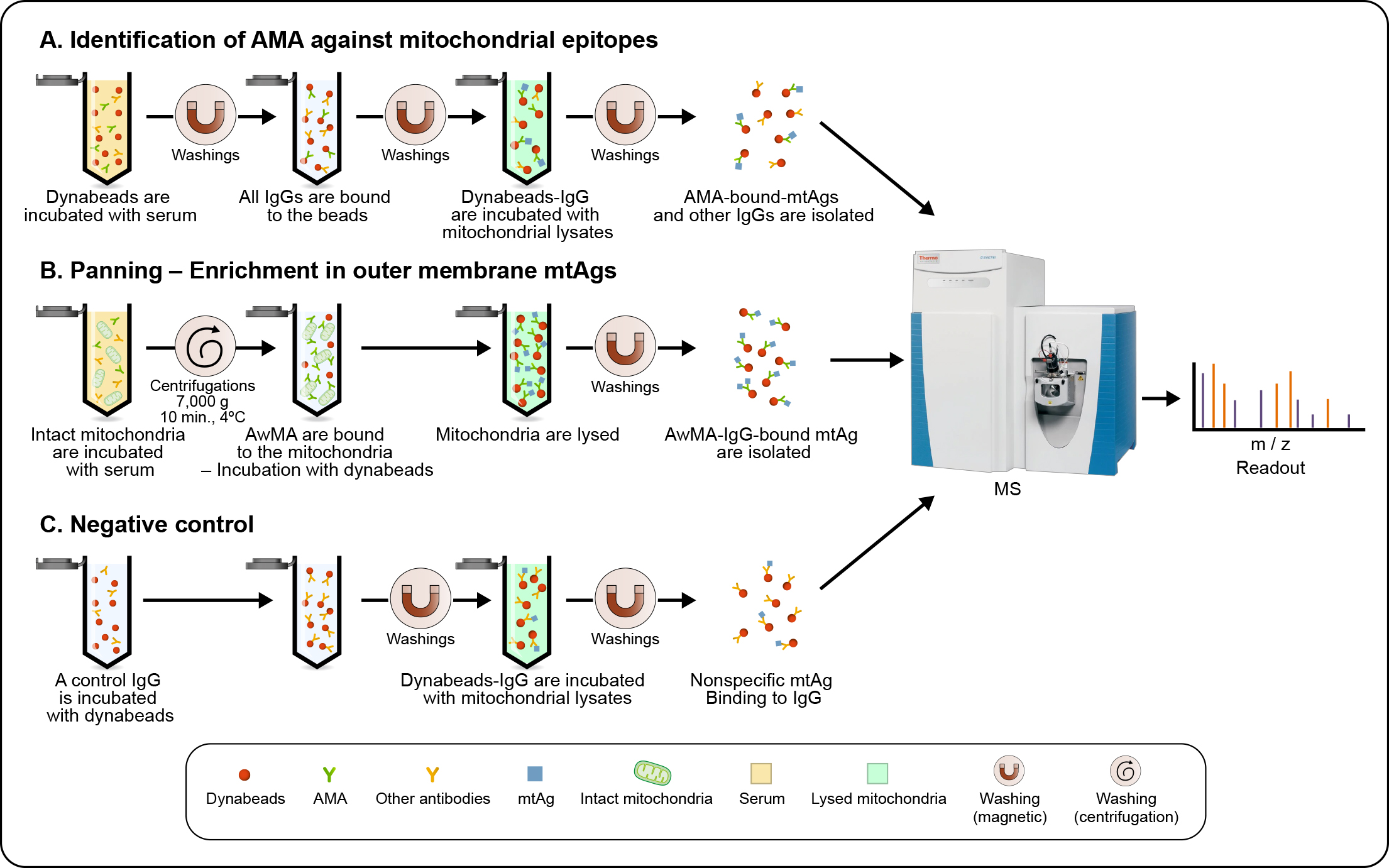 Figure 1: Workflow used for the detection of mitochondrial antigens targeted by anti mitochondrial antibodies in SLE. (a) Antibodies of the IgG subclass are isolated, using Dynabeads®-Protein G, from pooled sera from either 10 healthy donors or 10 SLE patients with high levels of anti-whole mitochondrial IgG (AwMA IgG). Dynabeads®-bound IgG were subsequently incubated with mitochondrial lysates, allowing the affinity purification of mitochondrial antigens (mtAgs) from all sub-localizations. (b) Freshly isolated intact mitochondria were incubated with pooled sera. Mitochondria incubated with AwMA were then lysed and AwMA-IgG isolated with Dynabeads™. (c) An irrelevant monoclonal IgG targeting FcɣRIIa – a protein absent from mitochondria, is bound to Dynabeads™ and incubated with mitochondrial lysates in order to identify non-specific binding of mtAgs. For each approach, samples were acquired in triplicate and mtAgs were identified by mass-spectrometry (MS).
Figure 1: Workflow used for the detection of mitochondrial antigens targeted by anti mitochondrial antibodies in SLE. (a) Antibodies of the IgG subclass are isolated, using Dynabeads®-Protein G, from pooled sera from either 10 healthy donors or 10 SLE patients with high levels of anti-whole mitochondrial IgG (AwMA IgG). Dynabeads®-bound IgG were subsequently incubated with mitochondrial lysates, allowing the affinity purification of mitochondrial antigens (mtAgs) from all sub-localizations. (b) Freshly isolated intact mitochondria were incubated with pooled sera. Mitochondria incubated with AwMA were then lysed and AwMA-IgG isolated with Dynabeads™. (c) An irrelevant monoclonal IgG targeting FcɣRIIa – a protein absent from mitochondria, is bound to Dynabeads™ and incubated with mitochondrial lysates in order to identify non-specific binding of mtAgs. For each approach, samples were acquired in triplicate and mtAgs were identified by mass-spectrometry (MS).
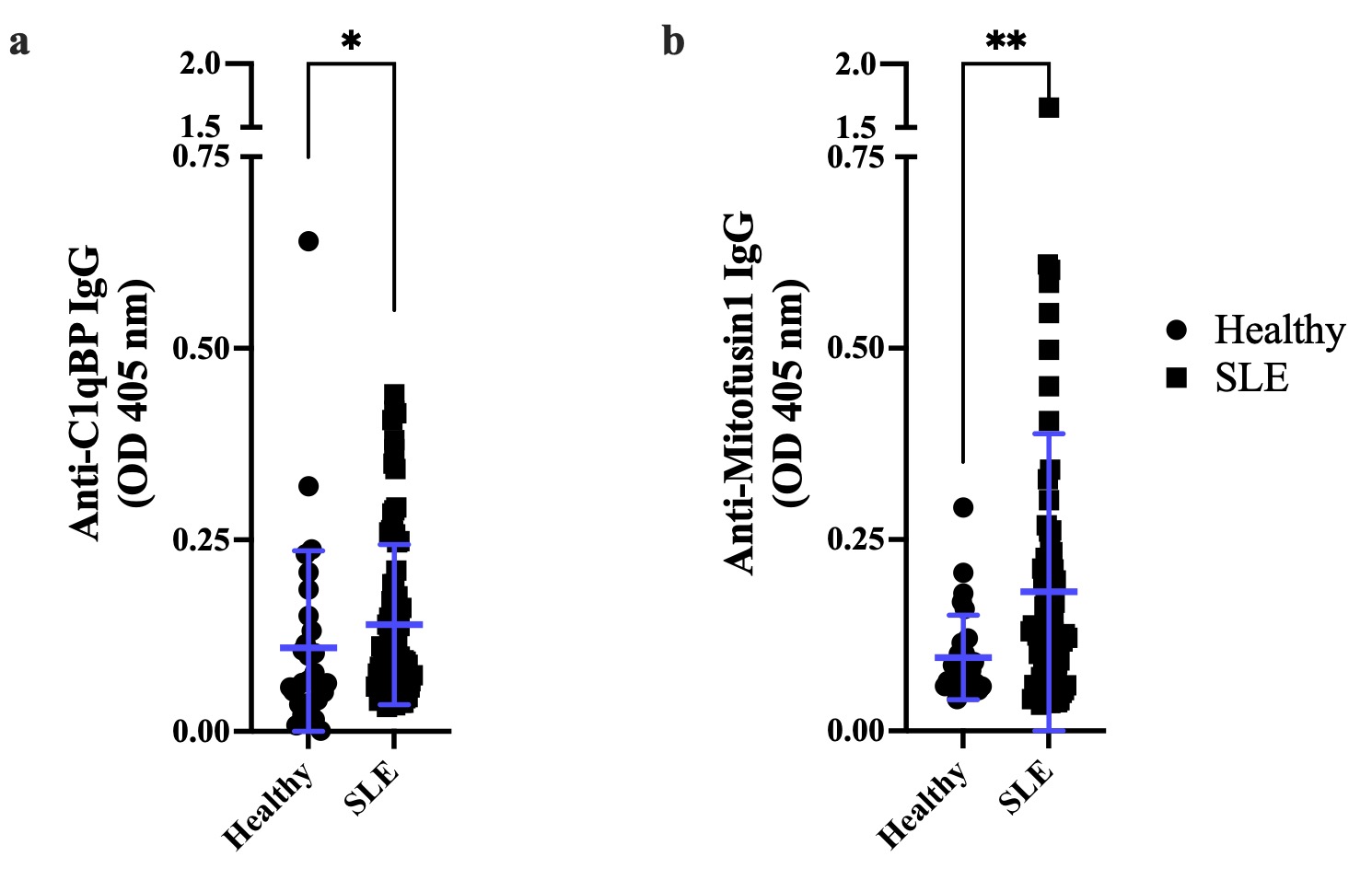 Figure 2: C1qBP and Mfn1 are two surface mtAgs with increased immunoreactivity in SLE. Immunoreactivity against mitochondrial proteins was assessed by direct ELISA. (a) The receptor for the complement component C1q (C1qBP) is a protein stored within the mitochondrion and subsequently dispatched to the cell surface and/or released into the extracellular space. SLE patients display increased levels of IgGs targeting C1qBP, compared with healthy individuals (p=0.0167). (b) Mitofusin 1 (Mfn1) is a protein expressed at the surface of the mitochondrion and is responsible for the fusion of mitochondrial outer membranes; anti Mfn 1 IgG are significantly increased in patients with SLE, compared with healthy donors (p=0.0022). Healthy: n=30, SLE: n=87. Data are mean optical densities read at 405 nm (OD405 nm) ± standard deviation. Wilcoxon-Mann-Whitney test. *: p≤0.05; **: p < 0.01.
Figure 2: C1qBP and Mfn1 are two surface mtAgs with increased immunoreactivity in SLE. Immunoreactivity against mitochondrial proteins was assessed by direct ELISA. (a) The receptor for the complement component C1q (C1qBP) is a protein stored within the mitochondrion and subsequently dispatched to the cell surface and/or released into the extracellular space. SLE patients display increased levels of IgGs targeting C1qBP, compared with healthy individuals (p=0.0167). (b) Mitofusin 1 (Mfn1) is a protein expressed at the surface of the mitochondrion and is responsible for the fusion of mitochondrial outer membranes; anti Mfn 1 IgG are significantly increased in patients with SLE, compared with healthy donors (p=0.0022). Healthy: n=30, SLE: n=87. Data are mean optical densities read at 405 nm (OD405 nm) ± standard deviation. Wilcoxon-Mann-Whitney test. *: p≤0.05; **: p < 0.01.
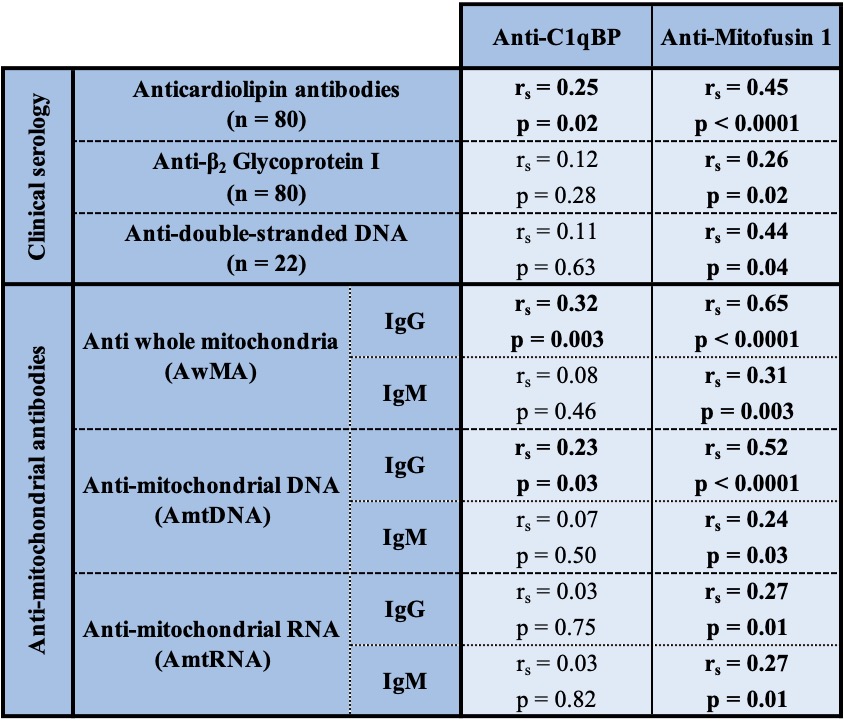 Table 1: Correlations between anti-C1qBP, anti-Mitofusin 1 and various continuous variables in SLE patients. Correlations presented, herein, for anticardiolipins or anti-β2-GPI were tested for IgGs.
Table 1: Correlations between anti-C1qBP, anti-Mitofusin 1 and various continuous variables in SLE patients. Correlations presented, herein, for anticardiolipins or anti-β2-GPI were tested for IgGs.
To cite this abstract in AMA style:
BECKER Y, Gagné J, Julien A, Lévesque T, Gougeard N, Rubio V, Boisvert F, Jean D, Poirier G, Fortin P, Boilard. Identification of Mitochondrial Antigens Targeted by Autoantibodies in Systemic Lupus Erythematosus (SLE) [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/identification-of-mitochondrial-antigens-targeted-by-autoantibodies-in-systemic-lupus-erythematosus-sle/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/identification-of-mitochondrial-antigens-targeted-by-autoantibodies-in-systemic-lupus-erythematosus-sle/
