Session Information
Date: Sunday, November 13, 2022
Title: Systemic Sclerosis and Related Disorders – Basic Science Poster
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Phagocytosis is a crucial cellular process, which under certain immuno-pathological conditions can be activated in macrophages in an uncontrolled manner. Biomarker studies have implicated macrophages in the development and progression of systemic sclerosis (SSc). Fos-like 2 (FOSL-2) transcription factor has been associated with altered macrophages in SSc. We postulate that macrophage phagocytosis is a critical process in SSc progression. The aim of this study was to investigate the role of FOSL-2 in macrophage phagocytosis in patients with SSc.
Methods: Published datasets of single cell RNA sequencing (scRNA-seq) of human explanted lung tissue from SSc-ILD1 and skin from dcSSc patients2 were analyzed using the the R package Seurat V2.3.4. Human monocyte-derived macrophages (hMDM) were differentiated from CD14+ blood-derived monocytes from healthy controls (HC) and SSc patients. hMDM were polarized with LPS (10 ng/ml) or remained unstimulated. Protein expression was detected by Western Blot. pHrodo Red E.coli particles were used to measure phagocytic activity, which was assessed by flow cytometry. hMDM were transfected with FOSL2 or negative control siRNA.
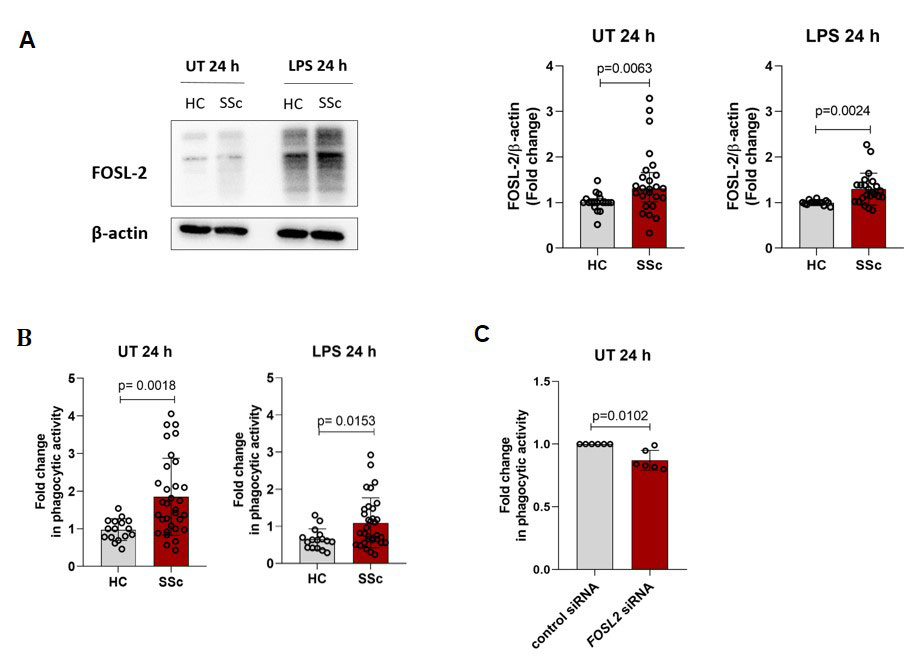
Results: scRNA-seq analyses of the SSc-ILD lung dataset identified differentially expressed phagocytosis-related genes (MARCO, C1QA, C1QB, C1QC) in FOSL2hi SPP1hi lung macrophages from SSc-ILD patients compared to FOSL2null cells (p.adj.≤0.05; log2 ratio≥0.5). Further, scRNA-seq analysis of dcSSc skin tissue revealed that FOSL2hi CCR1+ skin macrophages showed higher expression of phagolysosome-related genes (CORO1A, ARL8B) compared to FOSL2null cells (p.adj.≤0.05; log2 ratio≥0.5). In the in vitro differentiated hMDM, we observed increased FOSL-2 protein levels in unstimulated and pro-inflammatory LPS stimulated SSc hMDM compared to healthy hMDM (Figure 1A). In addition, phagocytic activity of pHrodo Red E.coli particles was increased in unstimulated and LPS stimulated SSc hMDM compared to healthy hMDM (Figure 1B). We did not observe any significant differences in phagocytic activity between early SSc, lcSSc and dcSSc hMDM. To further study the exact role of FOSL2, we used siRNA-specific knockdown of FOSL2 in unstimulated SSc hMDM, which led to reduced phagocytic activity compared to siRNA control hMDM (Figure 1C).
Conclusion: For the first time, we showed that FOSL-2 is a driver of newly identified pro-phagocytic macrophages and a crucial contributor of enhanced macrophage phagocytosis in patients with SSc.
References
1. Valenzi, E., et al., Ann Rheum Dis, 2019, 78(10): p. 1379-1387.
2. Xue, D., et al., Arthritis Rheumatol, 2022, 74(2) : p. 329-341.
To cite this abstract in AMA style:
Hukara A, Tabib T, Rudnik M, Distler O, Blyszczuk P, Lafyatis R, Kania G. Identification of a New Type of Pro-phagocytic Macrophages in Patients with Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/identification-of-a-new-type-of-pro-phagocytic-macrophages-in-patients-with-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/identification-of-a-new-type-of-pro-phagocytic-macrophages-in-patients-with-systemic-sclerosis/