Session Information
Date: Saturday, November 7, 2020
Title: SLE – Treatment Poster I
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Iberdomide is a high-affinity cereblon ligand that promotes ubiquitination and proteasomal degradation of Ikaros (IKZF1) and Aiolos (IKZF3), transcription factors linked to the genetic risk for systemic lupus erythematosus (SLE). Ikaros is required for development of B cells and plasmacytoid dendritic cells (pDC) and represses IL-2 transcription. Aiolos is a B-cell modulator and required for maturation of plasma cells. The pharmacokinetics, pharmacodynamics (PD), efficacy, and safety of oral iberdomide were evaluated in a phase 2b study in subjects with active autoantibody-positive SLE (NCT03161483).
Methods: Adult SLE patients (N=288) with a ≥6-month history of SLE and SLE Disease Activity Index (SLEDAI 2K) ≥6 were randomized to placebo (n=83) or iberdomide 0.15 mg QD (n=42), 0.3 mg QD (n=82), or 0.45 mg QD (n=81). Clinical response was determined by the SLE Responder Index 4 (SRI-4) at week 24 for all patients and within prespecified biomarker-enriched subsets based on expression of Ikaros, Aiolos, the Type 1 IFN signature (IFI27, IFI44, IFI44L, RSAD2), and other gene modules measured from fingerstick blood collected at baseline (Modular Immune Profile Test, DxTerity Diagnostics, Inc.). PD changes in whole blood leukocytes were measured by flow cytometry (Covance), T regulatory cells (Tregs) by epigenetic assay (EpiontisID, Epiontis GmbH), and plasma cytokines by ultra-sensitive cytokine assays (Erenna, EMD Millipore). PD data were reported as treatment differences from placebo in adjusted mean percent changes from baseline.
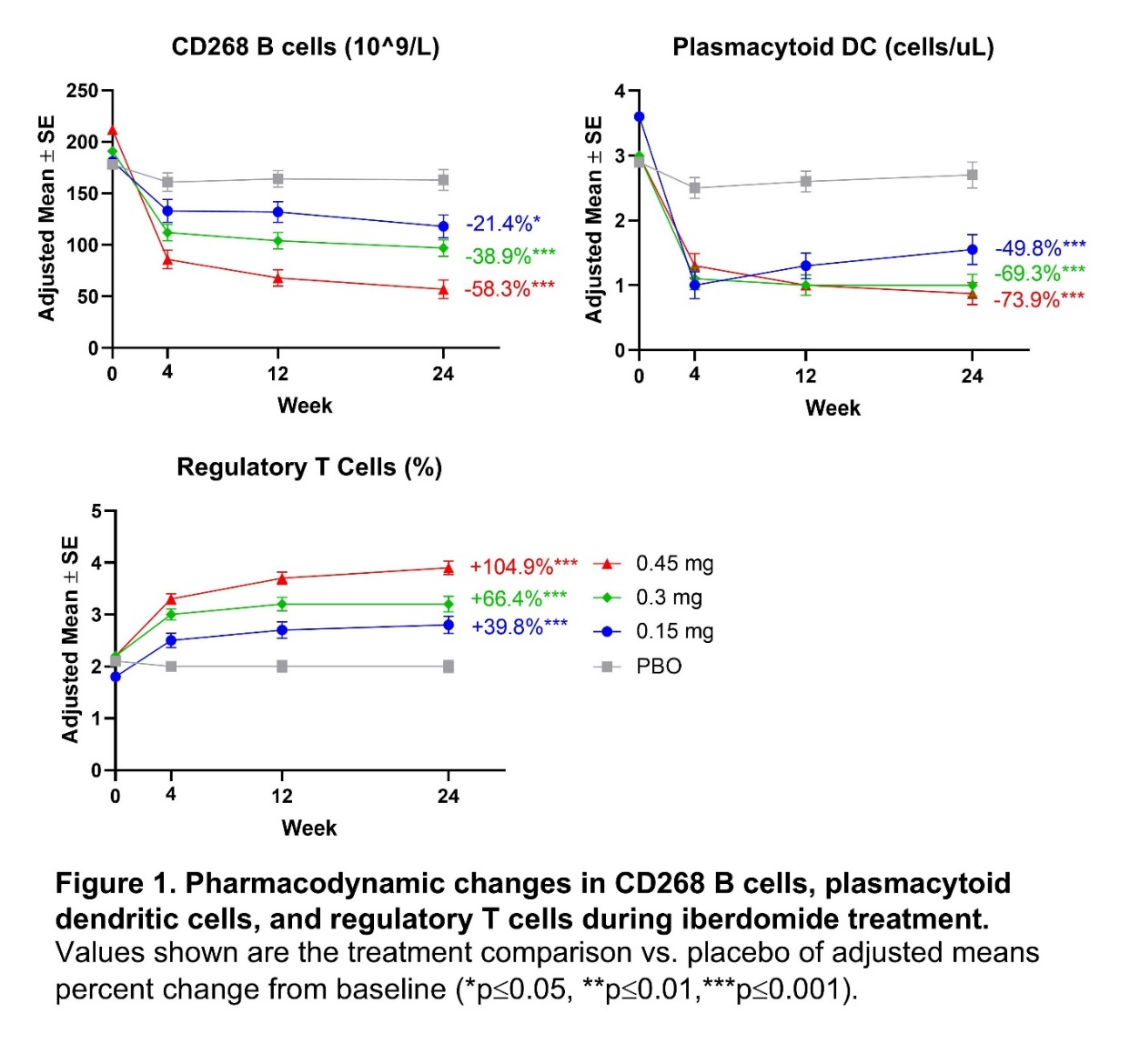
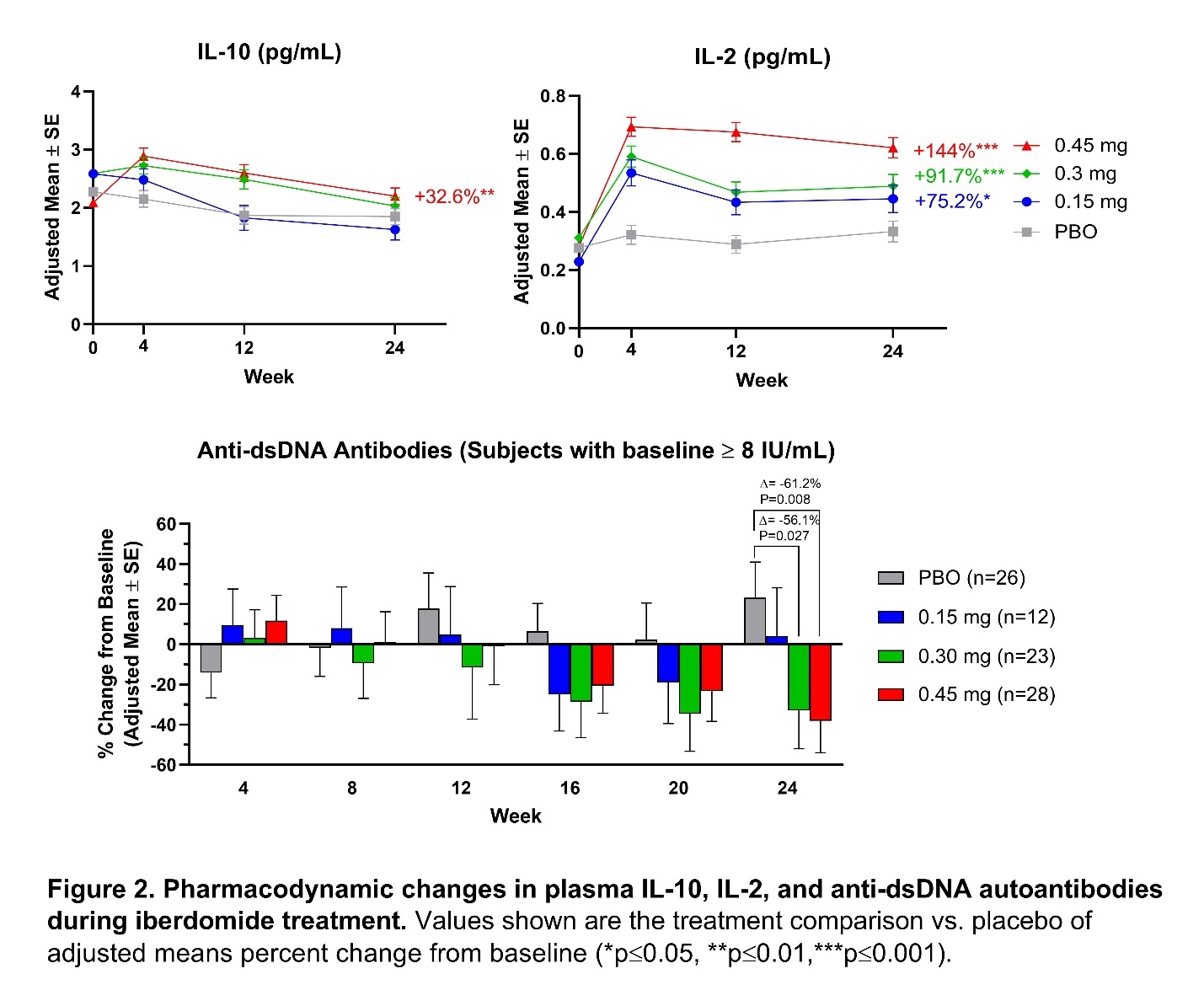
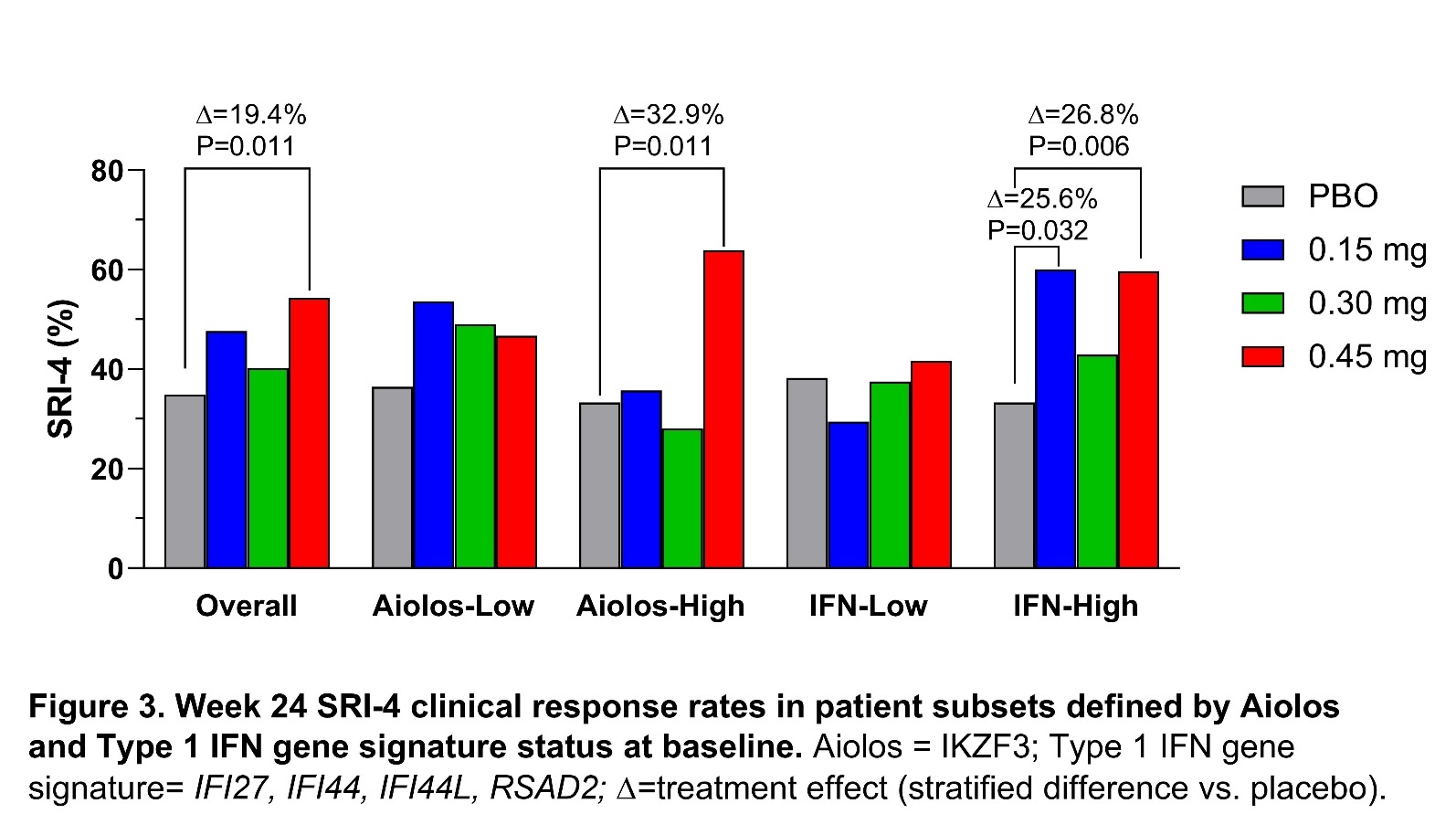
Results: Iberdomide pharmacokinetics were linear, with highest mean exposure observed at 0.45 mg. Iberdomide modulated leukocytes and cytokines in a dose-dependent manner, with significant changes at week 24 in the iberdomide 0.45 mg group compared with placebo, including decreased numbers of B cells, including those expressing CD268 (TNFRSF13C) (−58.3%; P< 0.001); decreased pDCs (−73.9%; P< 0.001); and increased Tregs (+104.9%; P< 0.001) (Figure 1). At week 24 compared with placebo, iberdomide 0.45 mg increased IL-10 (+32.6%; P=0.005) and IL-2 levels (+144.1%; P< 0.001) and decreased anti-double stranded (ds) DNA antibodies in patients with ≥8 IU/mL at baseline (−61.2%; P=0.008) (Figure 2). Iberdomide also decreased expression of the Type 1 IFN gene module. Iberdomide 0.45 mg caused a significant SRI-4 treatment effect versus placebo among patients within the baseline Aiolos-High subset (32.9%; P=0.011) and baseline Type 1 IFN-High subset (26.8%; P=0.006) (Figure 3).
Conclusion: Iberdomide significantly reduced activity of the Type 1 IFN and B cell/plasma cell pathways, including pDCs and Type 1 IFN-inducible genes as well as total B cells and those expressing TNFRSF13C, and anti-dsDNA antibody levels. Increases in Tregs, IL-2, and IL-10 suggest a broad rebalance of immune regulation. Clinical efficacy was noted in the entire cohort receiving 0.45 mg and was greater within the subsets with high expression of Aiolos or the Type 1 IFN gene signature at baseline. If validated, these PD effects may help refine future patient selection and dosing strategies for optimal use of iberdomide in SLE.
To cite this abstract in AMA style:
Lipsky P, van Vollenhoven R, Dörner T, Werth V, Merrill J, Furie R, Petronijevic M, Velasco Zamora B, Majdan M, Irazoque-Palazuelos F, Terbrueggen R, Delev N, Weiswasser M, Korish S, Agafonova N, Stern M, Hersey S, Ye Y, Gaudy A, Liu Z, Tang S, Schafer P. Iberdomide Decreases B Cells and Plasmacytoid Dendritic Cells, Increases Regulatory T Cells and IL-2, and Has Enhanced Clinical Efficacy in Active Systemic Lupus Erythematosus Patients with High Aiolos or the IFN Gene Expression Signature [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/iberdomide-decreases-b-cells-and-plasmacytoid-dendritic-cells-increases-regulatory-t-cells-and-il-2-and-has-enhanced-clinical-efficacy-in-active-systemic-lupus-erythematosus-patients-with-high-aiolo/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/iberdomide-decreases-b-cells-and-plasmacytoid-dendritic-cells-increases-regulatory-t-cells-and-il-2-and-has-enhanced-clinical-efficacy-in-active-systemic-lupus-erythematosus-patients-with-high-aiolo/