Session Information
Date: Monday, October 27, 2025
Title: (0897–0915) B Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: B cells are key players in the pathogenesis of Sjögren’s disease (SjD) and other systemic auto-immune diseases, supporting B cell depletion as an attractive therapeutic strategy in these patients. However, survival signals mediated by high levels of B cell-activating factor (BAFF) may interfere with B cell depletion, as well as drive disease flares. Ianalumab, an investigational afucosylated monoclonal Ab targeting BAFF-receptor (BAFF-R), has been shown to deplete B cells through enhanced antibody-dependent cellular cytotoxicity (ADCC) with concurrent blockade of BAFF:BAFF-R mediated signals [1]. Here, we extensively characterize the properties of ianalumab on B cells in vitro, as well as its ability to deplete circulating and tissue B cells in C57/B6 mice and in the spontaneous non-obese diabetic (NOD) mouse model of SjD.
Methods: In vitro B cell killing was assessed using isolated NK cells and B cells from healthy volunteers (HVs) and patients with SjD or SLE. In vitro blockade of BAFF stimulation was evaluated through Western blots of Nuclear Factor Kappa B Subunit 2 (NF-kB2) intact and cleaved forms, B cell proliferation measured by thymidine incorporation, and quantification of IgG secretion. The efficacy of B cell depletion following administration of ianalumab in C57/B6 and NOD mice was investigated using flow cytometry and/or histology in blood and relevant organs.
Results: In an ADCC assay co-culturing purified NK cells with B cells from HVs, ianalumab showed a 44-fold increased potency compared to rituximab (Fig. 1A). This increased potency was also observed when NK cells from patients with SjD and SLE were tested (Fig. 1B). Additionally, ianalumab effectively prevented BAFF from binding to BAFF-R expressing cells. This blockade of BAFF-R on human B cells correlated with effective inhibition of BAFF-induced cleavage of NF-κB2, proliferation and IgG production. Notably, ianalumab was able to inhibit B cell proliferation with the same potency, when induced by a BAFF trimer or 60-mer (Fig. 2). In vivo, ianalumab induced a significant reduction of B cell subpopulations in blood and lymphoid organs of B6 mice. Ianalumab was also able to prevent B cell infiltration in the salivary glands of NOD mice when given prophylactically and to decrease B cells infiltrating the salivary glands therapeutically, showing its ability to reduce B cells in the target organs of mice suffering from systemic autoimmunity (Fig. 3).
Conclusion: Ianalumab, through its dual mechanism of action, addresses limitations of first-generation B cell targeting therapies for auto-immune diseases by providing more potent B cell depletion and additional BAFF-R blockade on remaining B cells. Accordingly, patients with SLE (NCT03656562) or SjD (NCT02962895) treated with ianalumab for up to 52 weeks showed sustained reduction in disease activity in phase 2 trials [2,3]. Ongoing phase 3 studies in SjD, SLE and LN will provide further evidence on the efficacy and safety of ianalumab in larger patient populations.References:1. McWilliams EM, et al. Blood Adv. 2019;3:447-602. Shen N, et al. [abstract]. Arthritis Rheumatol. 2023;75 (suppl 9). 3. Bowman SJ, et al. Lancet. 2022;399:161-71.
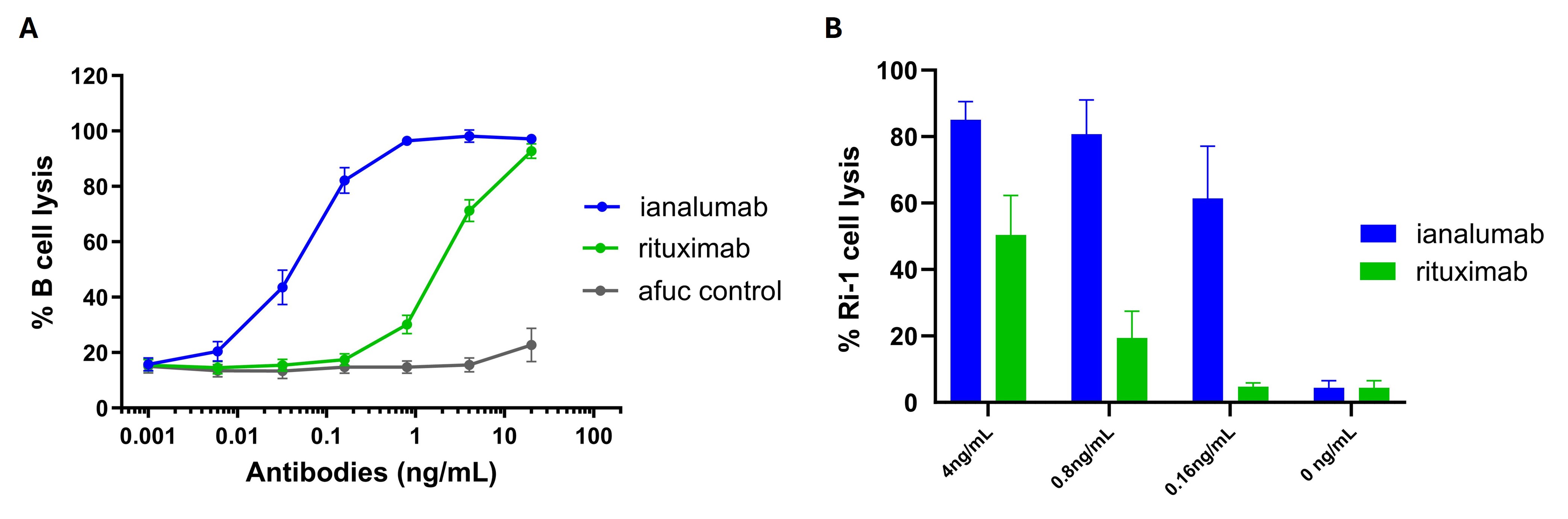 Figure 1. Ianalumab shows superior potency to rituximab in ADCC. A. B cells and NKs from HVs (Nf7 donors) where cocultured in the presence of ianalumab, rituximab or an irrelevant afucosylated antibody, and B cell lysis was evaluated after 60 min. B. NK cells from patients with SjD (Nf3 donors) were cocultured with RI-1 cells in the presence of ianalumab or rituximab and Ri-1 cell lysis was evaluated after 60 min.
Figure 1. Ianalumab shows superior potency to rituximab in ADCC. A. B cells and NKs from HVs (Nf7 donors) where cocultured in the presence of ianalumab, rituximab or an irrelevant afucosylated antibody, and B cell lysis was evaluated after 60 min. B. NK cells from patients with SjD (Nf3 donors) were cocultured with RI-1 cells in the presence of ianalumab or rituximab and Ri-1 cell lysis was evaluated after 60 min.
.jpg) Figure 2. Ianalumab inhibits proliferation of human B cells from HVs (Nf7 donors) stimulated by BAFF in combination with anti-IgM. Purified B cells were stimulated with BAFF 3-mer or BAFF 60-mer, in the presence of anti-IgM. Cell proliferation was measured by 3H-thymidine incorporation.
Figure 2. Ianalumab inhibits proliferation of human B cells from HVs (Nf7 donors) stimulated by BAFF in combination with anti-IgM. Purified B cells were stimulated with BAFF 3-mer or BAFF 60-mer, in the presence of anti-IgM. Cell proliferation was measured by 3H-thymidine incorporation.
.jpg) Figure 3. Ianalumab reduces infiltration of B cells in spleen and salivary glands of NOD SjD mice. A. Proportion of CD19+ cells analysed at 20 weeks in salivary glands using flow cytometry. B. CD45R+ B cells analysed on paraffin sections of submandibular salivary glands.
Figure 3. Ianalumab reduces infiltration of B cells in spleen and salivary glands of NOD SjD mice. A. Proportion of CD19+ cells analysed at 20 weeks in salivary glands using flow cytometry. B. CD45R+ B cells analysed on paraffin sections of submandibular salivary glands.
To cite this abstract in AMA style:
Wioland C, Vedrine C, Walter C, Marque F, Dannequin T, Cecci M, Buffet D, Schmid C, Degl'Innocenti E, Robert G, Wieczorek G, Schubert D, Paape C, Isnardi I. Ianalumab‘s dual mode of action: targeting B cells through enhanced B cell depletion and blockade of B cell-activating factor receptor signaling [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/ianalumabs-dual-mode-of-action-targeting-b-cells-through-enhanced-b-cell-depletion-and-blockade-of-b-cell-activating-factor-receptor-signaling/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ianalumabs-dual-mode-of-action-targeting-b-cells-through-enhanced-b-cell-depletion-and-blockade-of-b-cell-activating-factor-receptor-signaling/
