Session Information
Date: Monday, November 13, 2023
Title: (1200–1220) Patient Outcomes, Preferences, & Attitudes Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Systemic sclerosis (SSc) is a multisystem autoimmune rheumatic disease with heterogeneous manifestations, including common symptoms such as pain, fatigue, dyspnea, and Raynaud’s phenomenon. It can also include rare but life-threatening complications such as renal failure and pulmonary hypertension. While the ACR Composite Response Index in Systemic Sclerosis (ACR CRISS), an existing clinical outcome assessment (COA), has shown favorable performance in SSc clinical trials, research is needed to develop a COA to more fully assess the way a patient feels, functions, and/or survives, per the FDA guidance. The purpose of this research is to comprehensively identify the disease symptoms (DS) and treatment side effects (TSE) that are most bothersome for patients with SSc toward the development a new and robust COA.
Methods: Concept elicitation interviews were conducted from February 2023 to May 2023 with 28 participants with SSc (15 participants with diffuse cutaneous SSc and 13 participants with limited cutaneous SSc). The 90-minute interviews focused on DS and TSE, how bothersome they were (0 = not at all bothersome to 10 = extremely bothersome), and their impacts on participants’ lives. Interview data were summarized and results were analyzed thematically.
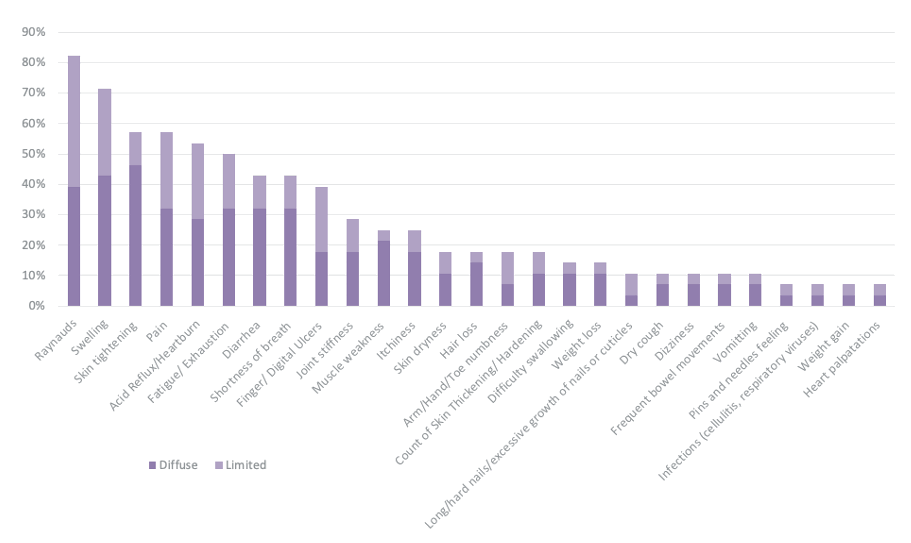
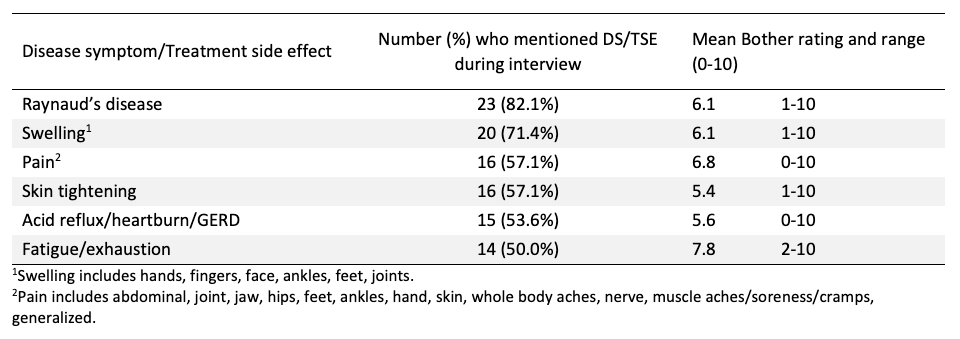
Results: The sample had a mean age of 54 years (range: 34-69) and was primarily women (82.1%), consistent with the broader gender distribution of SSc. The sample was diverse, including White (75.0%), Black (17.8%), Hispanic (7.1%), and American Indian (3.6%) participants. Overall, the sample reported 76 unique concepts (DS/TSE). After combining similar concepts for analytic purposes (e.g., sleep disturbances combined insomnia, hypersomnia) participants reported 66 DS/TSE. Participants with diffuse SSc reported an average of 13 DS/TSEs, while participants with limited SSc reported an average of 7 DS/TSEs. As presented in Figure 1, the most described DS/TSE across all participants was Raynaud’s disease (82.1%), followed by swelling (71.4%), pain (57.1%), skin tightening (57.1%), acid reflux/heartburn/GERD (53.6%), and fatigue/exhaustion (50%). Of those DS/TSEs, fatigue was described as the most bothersome (M=7.8), followed by pain (M=6.8), swelling (M=6.1), Raynaud’s (M=6.1), acid reflux/heartburn/GERD (M=5.6), and skin tightening (M=5.4). See Table 1 for more detail. Fatigue was the top bothersome DS/TSE for both diffuse and limited groups (M=7.8 and M=7.6 respectively). Both groups reported DS/TSE unique to their respective groups. Participants in the diffuse group uniquely reported contractures (46.7%), headaches (33.3%), skin discoloration (26.7%), and bloating/cramping (26.7%). Participants in the limited group uniquely reported eczema/red dots/blood vessels on skin (30.8%), and facial rash (15.4%).
Conclusion: These findings were consistent with symptoms described in the “Voice of the Patient” report for systemic sclerosis (2021) developed for the FDA’s Patient-Focused Drug Development Initiative. This research will aid the creation of a new SSc COA measure using existing FACIT, PROMIS, and Neuro-QoL measurement systems, and new SSc-specific items, which will be capable of assessing multiple patient-centered domains.
To cite this abstract in AMA style:
Perschon C, Jaeger E, Greene G, Lescoat A, Chen Y, Murphy S, Shaunfield S, Cella D, Khanna D. “I Call It Zombie Hands and Feet, That’s Actually How I Found out I Had This Disease”: Disease Symptoms and Treatment Side Effects in a Diverse Sample of Patients with Early Limited Cutaneous and Diffuse Cutaneous Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/i-call-it-zombie-hands-and-feet-thats-actually-how-i-found-out-i-had-this-disease-disease-symptoms-and-treatment-side-effects-in-a-diverse-sample-of-patients-with-early-limited-c/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/i-call-it-zombie-hands-and-feet-thats-actually-how-i-found-out-i-had-this-disease-disease-symptoms-and-treatment-side-effects-in-a-diverse-sample-of-patients-with-early-limited-c/