Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Development of reliable disease activity biomarkers is critical for diagnostics, prognostics, and novel drug development. In the case of preclinical models of inflammatory-erosive arthritis, such as sexually dimorphic tumor necrosis factor transgenic (TNF-Tg) mice (1), disease severity is routinely quantified in the ankle through manual segmentation of the talus or small regions of adjacent bones primarily due to the ease in measurement (2,3). Herein, we sought to determine the particular hindpaw bones that represent reliable biomarkers of sex-dependent disease progression to guide future investigation and analysis.
Methods: Hindpaw micro-computed tomography (μCT) was performed on wild-type (n=4 male, n=4 female) and TNF-Tg (n=4 male, n=7 female) mice at monthly intervals from 2-5 (females) and 2-8-months (males) of age, where female TNF-Tg mice exhibit early mortality from cardiopulmonary disease at approximately 5-6-months (1). For image analysis, we utilized our recently developed high-throughput and semi-automated segmentation strategy in Amira software (v2020.2) (4). Synovial (H&E-OG) and osteoclast (TRAP) areas of ankle joints were quantified using Visiopharm (v2021.07).
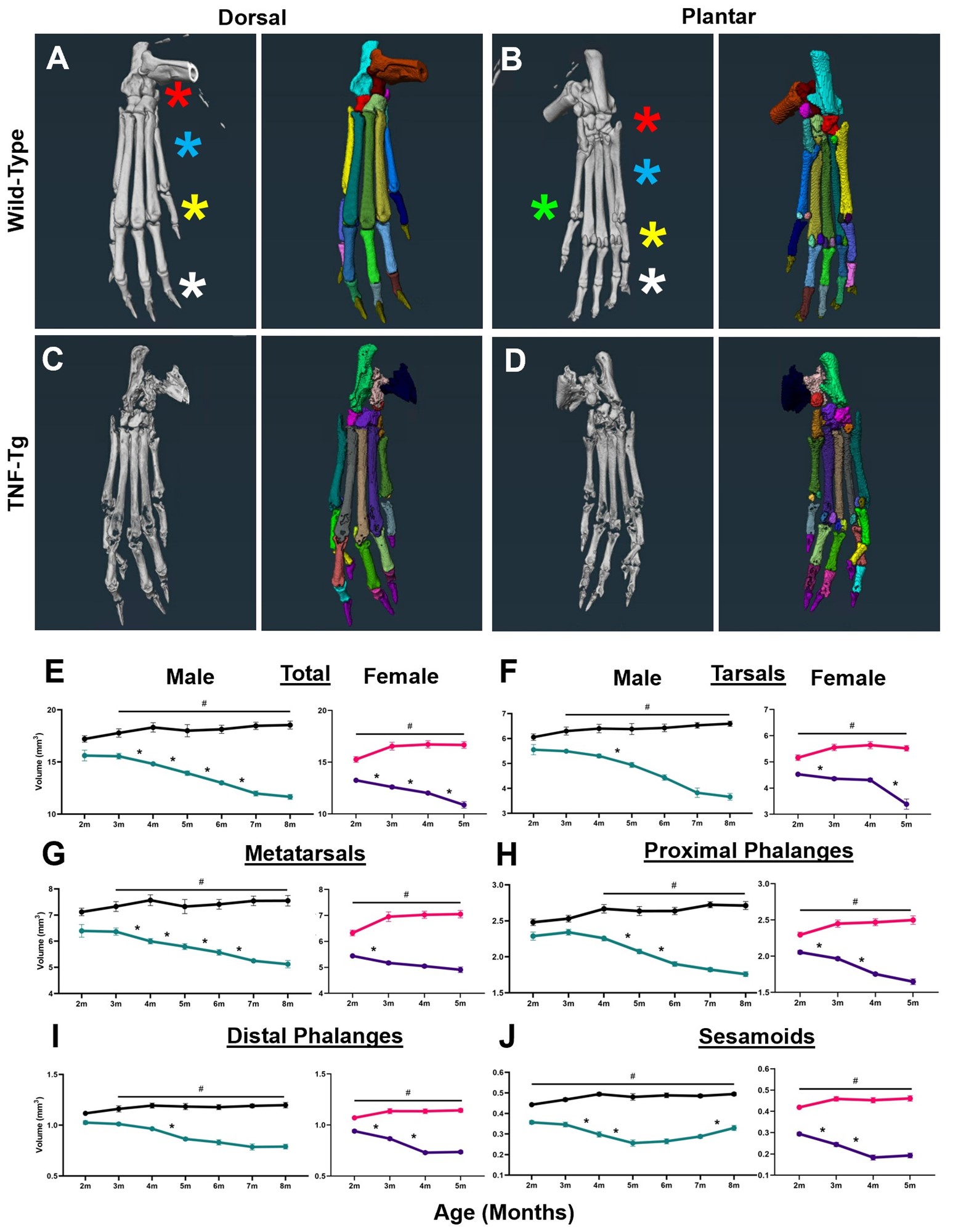
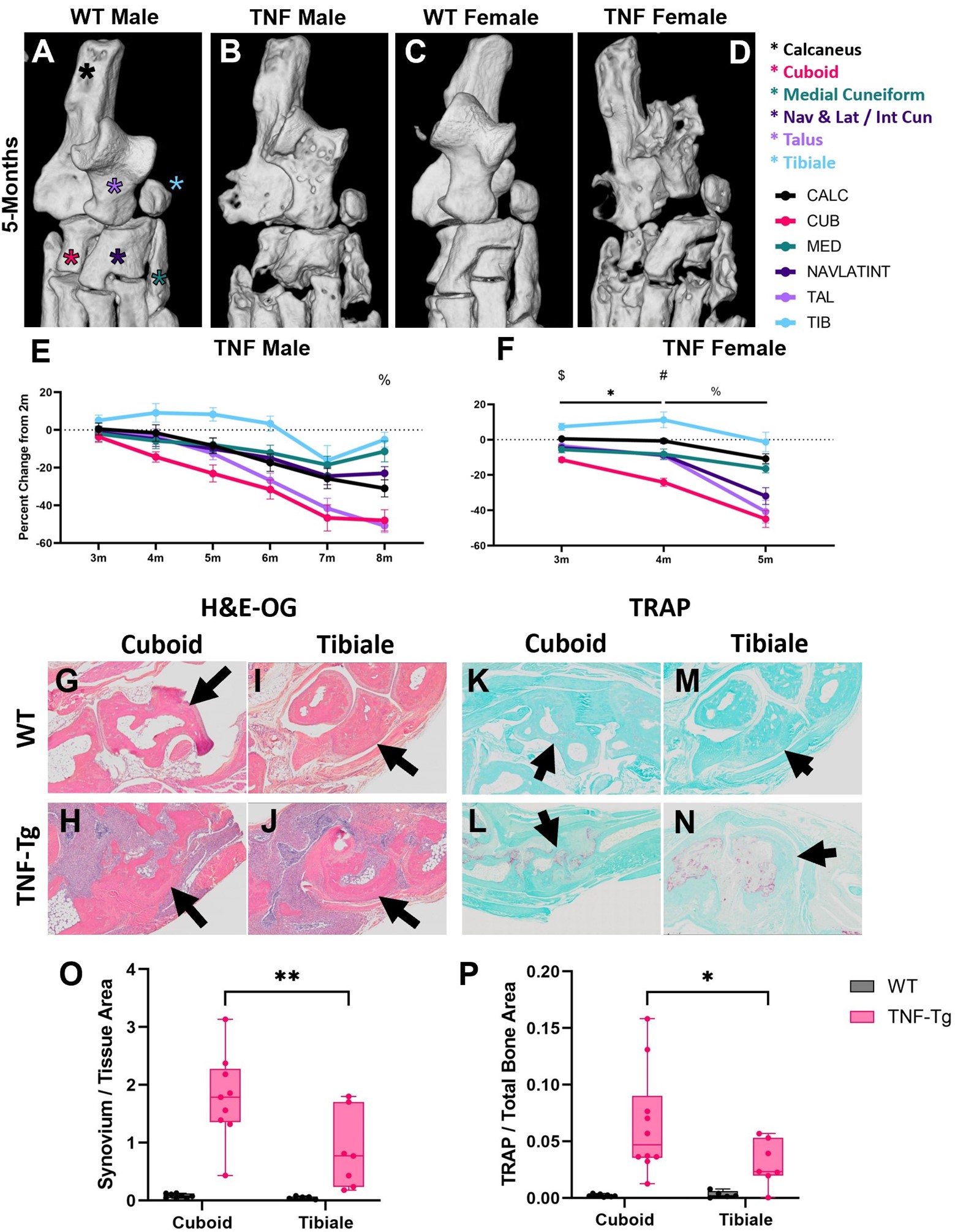
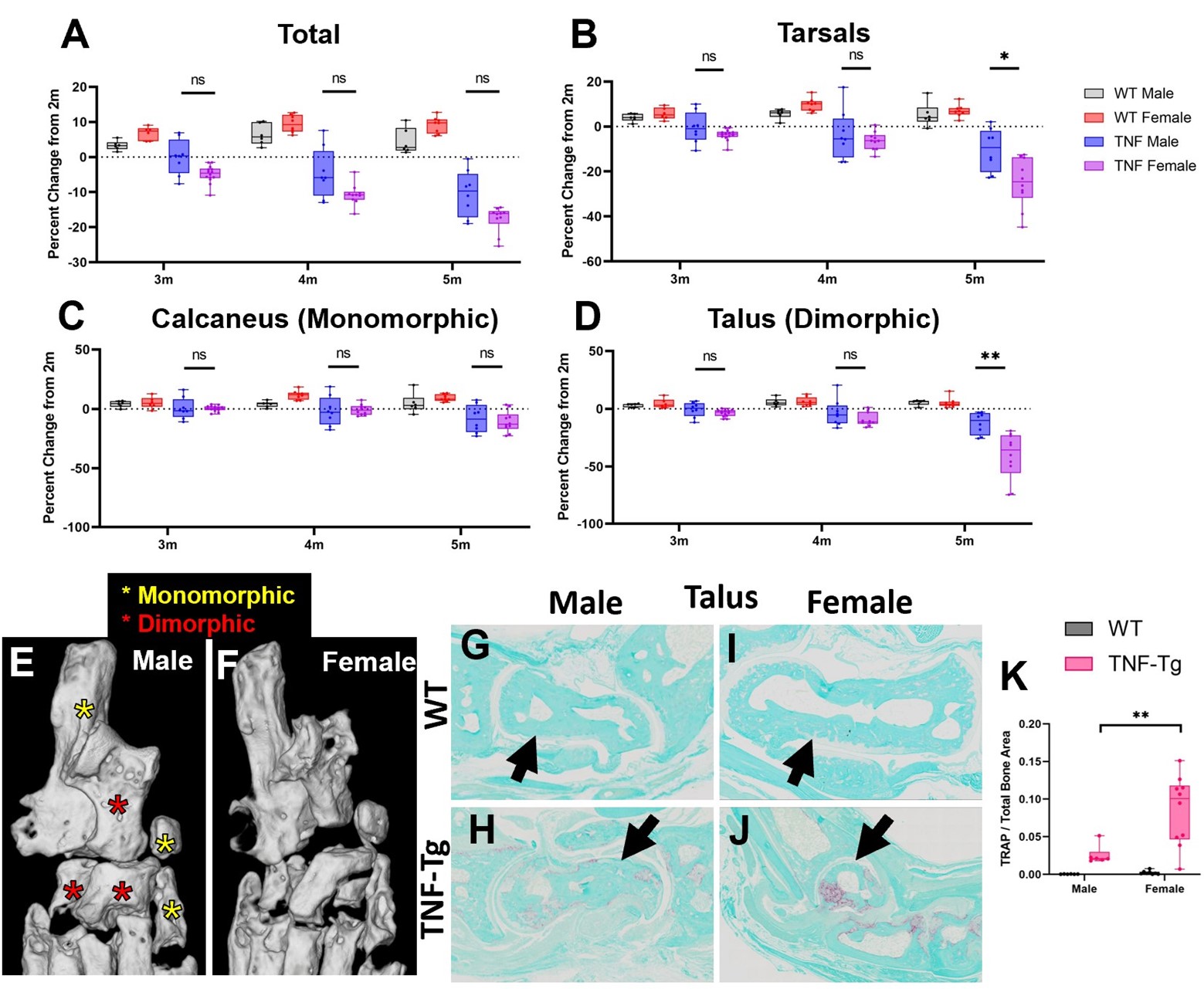
Results: First, we demonstrated our analysis method had comparable automated segmentation accuracy in wild-type and TNF-Tg hindpaws before correction (79.2±8.9% vs 80.1±5.1%, p=0.52), determined through analysis of ~9000 individual bones by a single user, with representative μCT (left) and bone segmentation (right) provided (Fig. 1A-D). Compared to other bone compartments, the tarsal region demonstrated a sudden, specific, and significant bone volume reduction in female TNF-Tg mice by 5-months (Fig. 1E-J). This sexual dimorphism was associated with unique bone-specific changes across time, as the cuboid at 4-months of age showed significantly reduced bone volumes compared to all other tarsals. In contrast, TNF-Tg male mice exhibited no difference between individual bone volumes at this timepoint (Fig. 2A-F). Compared to bones with limited erosions (i.e., tibiale), the cuboid showed a corresponding increased synovial and TRAP area (Fig. 2G-P). At 5-months of age, additional bones localized to the antero-lateral region of the ankle were also responsible for the dramatic erosions in the tarsal region of females (Fig. 3A-F, red stars), coinciding with increased TRAP+ osteoclasts in female vs male TNF-Tg mice (Fig. 3G-K).
Conclusion: Taken together, here we demonstrated that sexual dimorphism of arthritis in TNF-Tg mice is bone-specific, where the cuboid serves as a reliable biomarker of erosive arthritis with the greatest sensitivity to early and consistent bone loss related to enhanced osteoclast numbers. Ongoing work will further investigate the cellular mechanisms of these bone-specific erosions in mice and evaluate the translational potential of these biomarkers in arthritis patients through our segmentation model. 1. Bell et al. Arthritis Rheumatol. 71(9):1512-1523. 2019. 2. Proulx et al. Arthritis Rheumatol. 56(12):4024-4037. 2007. 3. Cambre et al. Nat Commun. 9(1):4613. 2018. 4. Kenney et al. Bone Rep. 16(101167). 2022.
To cite this abstract in AMA style:
Kenney H, Chen K, Schnur L, Fox J, Wood R, Xing L, Ritchlin C, Rahimi H, Schwarz E, Awad H. High-Throughput Semi-Automated Micro-CT Analysis Identifies the Cuboid Bone as a Sex-Dependent Biomarker of Inflammatory-Erosive Arthritis in TNF-Tg Mice [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/high-throughput-semi-automated-micro-ct-analysis-identifies-the-cuboid-bone-as-a-sex-dependent-biomarker-of-inflammatory-erosive-arthritis-in-tnf-tg-mice/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/high-throughput-semi-automated-micro-ct-analysis-identifies-the-cuboid-bone-as-a-sex-dependent-biomarker-of-inflammatory-erosive-arthritis-in-tnf-tg-mice/