Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Atherosclerosis involves both dyslipidemia and chronic inflammatory disease; however, lipid-lowering agents do not completely eliminate the risks of cardiovascular events. In addition, chronic systemic inflammatory diseases such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) are complicated by atherosclerosis. This mutual relationship suggests that a shared physiological pathway might regulate inflammatory responses in both atherosclerosis and inflammatory diseases. Interestingly, spleen tyrosine kinase (SYK), an enzyme abundantly expressed in hematopoietic cells, is involved in atherosclerosis, RA, and SLE. We aimed to identify a shared therapeutic target related to Syk for atherosclerosis and inflammatory diseases.
Methods: To determine whether SYK is involved in atherosclerosis through the inflammatory response and elucidate the mechanism of SYK signaling, we utilized Syk-knockout atherosclerosis-prone mice. Cell migration capacity was assessed using wound scratch and transwell migration assays. RNA-seq, flow cytometry, and adhesion assay were performed to identify a cell migration-related gene. We investigated the transcription factor that regulates the identified cell migration-related gene using reporter assays and flow cytometry. Additionally, we identified a molecule downstream of SYK signaling that mediates the transcription factor’s activity using flow cytometry. Furthermore, we evaluated whether the inhibition of the transcription factor could suppress the identified cell migration-related gene in monocyte and ameliorate atherosclerosis.
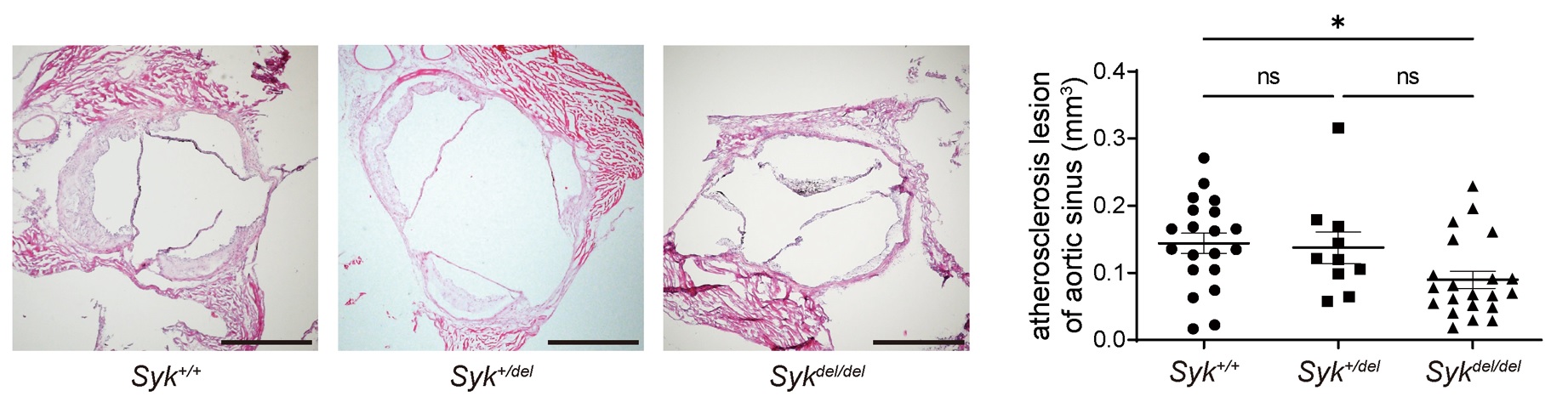
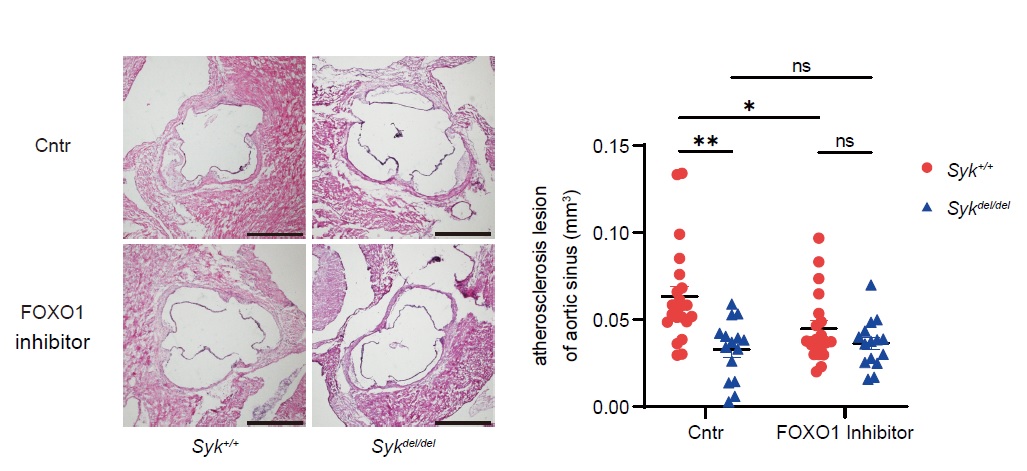
Results: Our results revealed that Syk-knockout mice exhibited reduced atherosclerosis. Wound scratch and transwell migration assays showed reduced cell migration in Syk-knockout bone marrow-derived macrophages (BMDM). RNA-seq analysis demonstrated decreased expression of CD11c, an adhesion molecule involved in inflammatory cell infiltration, in Syk-knockout bone marrow monocytes. Peripheral monocytes from Syk-knockout mice also exhibited reduced CD11c expression. Bocking CD11c protein resulted in inhibited adhesion capacity. A reporter assay showed SYK associated with approximately 1,000 upstream of the CD11c transcription starting site. The in silico analysis predicted forkhead box protein O1 (FOXO1) might bind to the predicted sequences by the reporter assay. The inhibitor of FOXO1 suppressed the CD11c expression of granulocyte–macrophage colony-stimulating factor receptor (GM-CSF) primed BMDM using flow cytometry. In addition, the inhibition of c-Jun amino-terminal kinase (JNK) suppressed the CD11c expression of GM-CSF primed BMDM. Finally, the FOXO1 inhibitor reduced the CD11c expression of bone marrow and peripheral monocyte and mitigated atherosclerosis in mice.
Conclusion: We discovered the GM-CSF receptor/SYK/JNK/FOXO1/CD11c signaling pathway. FOXO1 could be a novel potential therapeutic target for atherosclerosis and inflammatory diseases.
To cite this abstract in AMA style:
Tsukui D, Yoshitaka K, Kono H. GM-CSF receptor/SYK/JNK/FOXO1/CD11c Signaling Enhances Cell Migration to Promote Atherosclerosis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/gm-csf-receptor-syk-jnk-foxo1-cd11c-signaling-enhances-cell-migration-to-promote-atherosclerosis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/gm-csf-receptor-syk-jnk-foxo1-cd11c-signaling-enhances-cell-migration-to-promote-atherosclerosis/