Session Information
Date: Sunday, November 17, 2024
Title: Innate Immunity Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The primary objective of this study was to generate knock-in mice with the M694I variant of the human MEFV gene, a critical variant in the pathogenesis of familial Mediterranean fever (FMF), and analyze the resultant pathophysiological changes. Additionally, we aimed to explore the molecular mechanisms of splenocytes through single-cell RNA sequencing (scRNA-seq) and gene set enrichment analysis (GSEA).
Methods: M694I knock-in mice were generated using the CRISPR/Cas9 gene editing system. The insertion of oligo DNA was confirmed using Sanger sequencing. The study involved monitoring the survival rate and growth curves of mice, collecting peritoneal macrophages, and stimulating them with lipopolysaccharide (LPS) and ATP to analyze inflammatory responses. Flow cytometry was used to evaluate the cellular composition of the spleen, and cytokine levels were measured in serum samples. Furthermore, scRNA-seq was performed on splenocytes, followed by GSEA to identify the activated pathways in different cell populations.
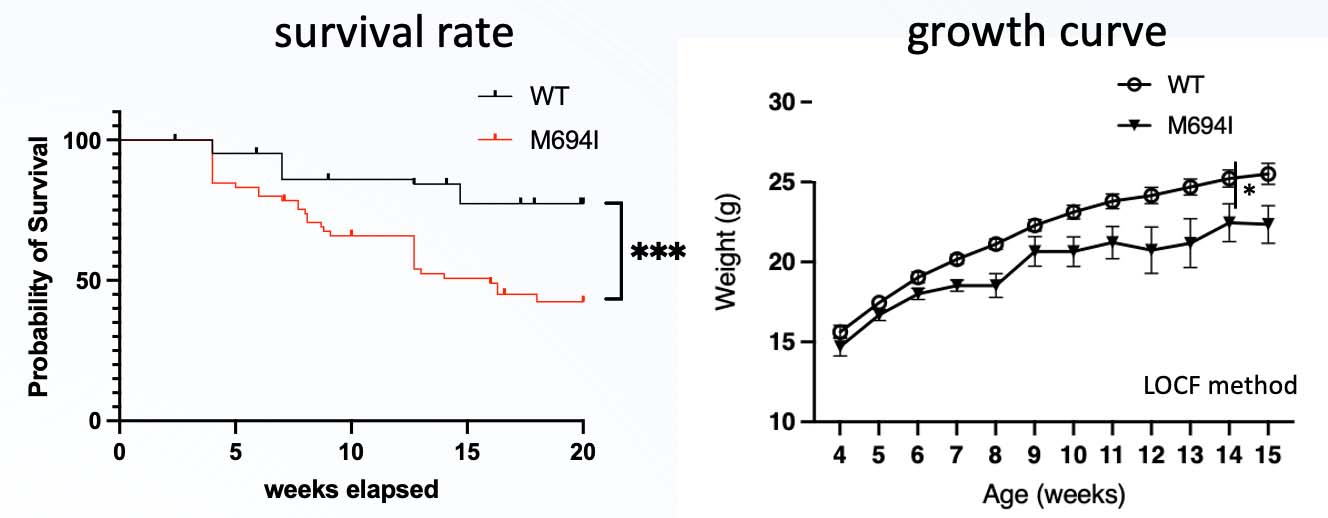
Results: The M694I group exhibited a significantly lower survival rate and failure to gain weight than the wild-type (WT) group, suggesting an inflammatory pathology. Peritoneal macrophages from the M694I group showed increased activation after LPS and ATP stimulation. M694I mice showed enlarged spleens and increased splenocyte counts, indicating an abnormal immune response. Serum analysis revealed elevated G-CSF, IFN-γ, IL-6, TNF-α, and IL-5 levels in the M694I group. Notably, a significant increase in IL-17 producing cells. The M694I mutation leads to a higher percentage of IL-17 producing cells, suggesting a role in Th17 cell differentiation. Additionally, scRNA-seq and GSEA of splenocytes revealed that T cells in M694I knock-in mice exhibited increased activity of HALLMARK_INTERFERON_ALPHA_RESPONSE and HALLMARK_INTERFERON_GAMMA_RESPONSE, whereas monocytes showed elevated activity of HALLMARK_TNFA_SIGNALING_VIA_NFKB.
Conclusion: Knock-in of the MEFV gene M694I variant in mice induced chronic inflammation resembling FMF. These findings suggest that Th17 cell and cytokine dysregulation play significant roles in the pathogenesis of FMF. The M694I mouse model is a valuable tool for studying the underlying mechanisms of FMF and evaluating potential therapeutic interventions. scRNA-seq analysis provided further insights into the molecular mechanisms, highlighting the involvement of interferon responses in T cells and TNFα signaling in monocytes. Further research is needed to explore the precise pathways involved and develop targeted treatments for FMF.
To cite this abstract in AMA style:
Koga T, Tsuji Y, Kawakami A. Generation and Pathophysiological Analysis of M694I Variant Knock-in Mice of Human MEFV Gene: Insights from Single-Cell RNA Sequencing [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/generation-and-pathophysiological-analysis-of-m694i-variant-knock-in-mice-of-human-mefv-gene-insights-from-single-cell-rna-sequencing/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/generation-and-pathophysiological-analysis-of-m694i-variant-knock-in-mice-of-human-mefv-gene-insights-from-single-cell-rna-sequencing/