Session Information
Date: Monday, October 27, 2025
Title: (0978–1006) T Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The absence of regulatory T cells (Tregs) results in multiorgan autoimmunity in the context of monogenic “Tregopathies,” but their role in mediating polygenic autoimmune diseases remains less clearly defined. Antigen-experienced human T cells expressing FoxP3, a transcription factor driving regulatory function, can be divided into FoxP3Lo and FoxP3Hi subsets. FoxP3Lo T cells are expanded in the context of autoimmunity, but there is a paucity of data defining the function of FoxP3Lo T cells or their relevance to immune-mediated fibrosis. Previous studies suggest they are non-suppressive and primarily composed of activated effector T cells. We examined FoxP3+ T cells across three different immune-mediated fibrotic diseases, systemic sclerosis (SSc), IgG4-related disease (IgG4-RD), and sarcoidosis.
Methods: We used spectral flow cytometry to examine circulating Treg subsets in PBMCs from patients with SSc (n=18, median age 55; 10 diffuse, 7 limited, 1 sine), IgG4-RD (n=27, median age 65, 15 head and neck, 10 pancreatobiliary, 2 retroperitoneal fibrosis, 6 kidney), sarcoidosis (n=17, median age 66, 7 only pulmonary, 10 extra-pulmonary involvement) and healthy donors (HD, n=17, median age 64). Bulk RNA sequencing of flow purified T cell populations was used to define the transcriptional differences between Treg subsets. Purified Treg subsets were used for in vitro assays to measure the capacity to suppress T cell proliferation.
Results: FoxP3Lo T cells were preferentially expanded in SSc and IgG4-RD (Fig 1C). FoxP3Lo T cell expansion correlated with disease severity, including early disease, skin score and interstitial lung disease in SSc and internal organ involvement in IgG4-RD (Fig 1 D-H). Expression of the transcription factor Helios reliably excluded IL2 and IFNg-producing effector T cell contaminants from the FoxP3Lo gate, as has been previously suggested (Fig 3A). Helios+ FoxP3Lo T cells are also expanded in SSc and IgG4-RD, indicating that the FoxP3Lo expansion is not driven by activated effector T cells (Fig 3C). FoxP3Lo T cells expressed decreased levels of the suppressive protein, CTLA4, and activation marker, ICOS, relative to FoxP3Hi cells, as previously reported. Transcriptionally, CD127LoCD25Lo (analogous to FoxP3Lo) T cells expressed decreased levels of the activation markers TNFRSF9 (CD137), IL2R (CD25), TNFRSF8 (CD30), CD70, and HLA-DR, as well as PTPN22, relative to CD25Hi cells, suggesting a difference in TCR binding affinity or activation state between these subsets (Fig 2B). The inhibitory receptor, PDCD1 (PD1), was uniquely upregulated in CD25Lo T cells. Despite differences in activation status and expression of CTLA4, CD127LoCD25Lo T cells showed an equal ability to suppress T cell proliferation in vitro when compared to their CD25Hi counterparts (Fig 3D).
Conclusion: We show that FoxP3+ T cells expressing low levels of FoxP3 are legitimate regulatory T cells but have reduced expression of certain markers of activation and Treg function. FoxP3Lo T cells are expanded in the immune-mediated fibrotic diseases SSc and IgG4-RD and correlate with disease severity, suggesting either a pathogenic role in the context of fibrosis or an induced regulatory response.
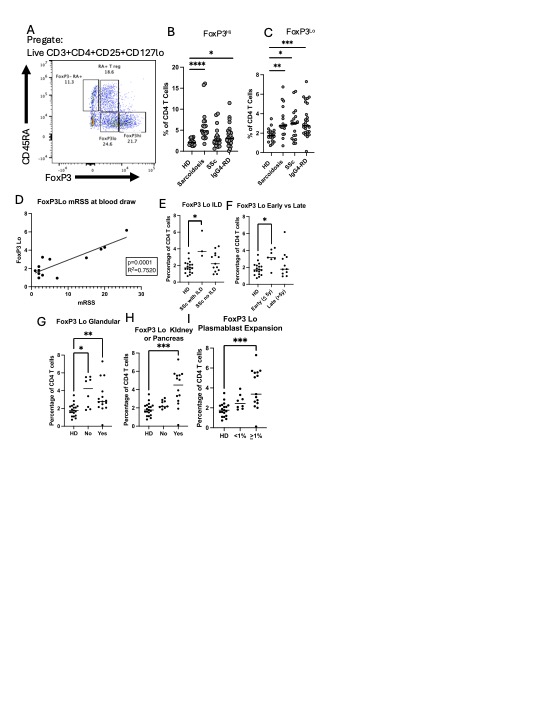 A representative flow plot of FoxP3/CD45RA subsets (pregated on live CD3+CD4+ CD25+CD127lo) are shown in (A). Graph of Treg subsets including FoxP3Hi (B, live CD3+CD4+ CD25+CD127lo CD45RA-FoxP3Hi) and FoxP3Lo (C, live CD3+CD4+ CD25+CD127lo CD45RA-FoxP3Lo). FoxP3Lo as a percentage of CD4+ T cells are graphed compared to modified Rodnan skin score (mRSS) (D). FoxP3Lo as a percentage of CD4+ T cells are graphed for SSc patients with and without ILD (E), and in early ( < 5 years from diagnosis)and late disease (>5 years from diagnosis) (F). FoxP3Lo as a percentage of CD4+ T cells are graphed for IgG4RD patients with and without parotid/salivary gland involvement (G), with and without kidney or pancreas involvement (H), and with and without plasmablast expansion, defined as >1% (I). For B-I: *p < 0.05, **p < 0.01, *** p < 0.001, **** p < 0.0001 by Mann-Whitney U test.
A representative flow plot of FoxP3/CD45RA subsets (pregated on live CD3+CD4+ CD25+CD127lo) are shown in (A). Graph of Treg subsets including FoxP3Hi (B, live CD3+CD4+ CD25+CD127lo CD45RA-FoxP3Hi) and FoxP3Lo (C, live CD3+CD4+ CD25+CD127lo CD45RA-FoxP3Lo). FoxP3Lo as a percentage of CD4+ T cells are graphed compared to modified Rodnan skin score (mRSS) (D). FoxP3Lo as a percentage of CD4+ T cells are graphed for SSc patients with and without ILD (E), and in early ( < 5 years from diagnosis)and late disease (>5 years from diagnosis) (F). FoxP3Lo as a percentage of CD4+ T cells are graphed for IgG4RD patients with and without parotid/salivary gland involvement (G), with and without kidney or pancreas involvement (H), and with and without plasmablast expansion, defined as >1% (I). For B-I: *p < 0.05, **p < 0.01, *** p < 0.001, **** p < 0.0001 by Mann-Whitney U test.
.jpg) T cell subsets from 7 healthy donors were sorted using flow cytometry by gating on live CD3+CD4+ cells. For Treg subsets: CD25+CD127Lo cells were gated on CD45RA+ population (CD45RA+Treg), CD45RA-CD25Lo(CD25Lo Treg; analagous to FoxP3Lo), and CD45RA- CD25Hi (CD25HiTreg; analagous to FoxP3Hi). For conventional T cell subsets, CD127+ and CD25-cells were gated on CD45RA+CCR7+ (Conv Naïve) or CD45RA-CCR7- (Conv Effector). RNA was isolated and bulk RNA sequencing was performed from different subsets. (A) A PCA plot was used to compare populations. (B) EBSeq was used to identify genes that are differentially expressed across the cell types with a posterior probability > 0.95. Select genes related to immune function are graphed for differentially regulated (DEG) upregulated in CD25Hi compared to CD25Lo(top), upregulated in CD25Lo compared to CD25Hi (middle), and upregulated in CD25Lo compared to Effector T cells.
T cell subsets from 7 healthy donors were sorted using flow cytometry by gating on live CD3+CD4+ cells. For Treg subsets: CD25+CD127Lo cells were gated on CD45RA+ population (CD45RA+Treg), CD45RA-CD25Lo(CD25Lo Treg; analagous to FoxP3Lo), and CD45RA- CD25Hi (CD25HiTreg; analagous to FoxP3Hi). For conventional T cell subsets, CD127+ and CD25-cells were gated on CD45RA+CCR7+ (Conv Naïve) or CD45RA-CCR7- (Conv Effector). RNA was isolated and bulk RNA sequencing was performed from different subsets. (A) A PCA plot was used to compare populations. (B) EBSeq was used to identify genes that are differentially expressed across the cell types with a posterior probability > 0.95. Select genes related to immune function are graphed for differentially regulated (DEG) upregulated in CD25Hi compared to CD25Lo(top), upregulated in CD25Lo compared to CD25Hi (middle), and upregulated in CD25Lo compared to Effector T cells.
.jpg) CD4 T cells were stimulated with PMA/Ionomycin for 4 hours and stained for intracellular FoxP3, IFNg, and IL2 (A). A representative plot of Helios gating is shown (B). (C) FoxP3Lo Helios+ T cells are pregated on Live CD3+CD4+CD127LoCD25+ and graphed as a percentage of total CD4+ T cells. (D) Conventional effector T cells were stimulated with CD3/CD28 dynabeads for 2 days then incubated at indicated ratios with FACS sorted CD45RA+ Tregs for 3 days (RA, CD3+CD4+CD127LoCD25+CD45RA+), CD25Lo Tregs (25Lo, CD3+CD4+CD127LoCD25LoCD45RA-), CD25Hi Tregs (25Hi, CD3+CD4+CD127LoCD25HiCD45RA-); proliferation as measured by Cell trace dye dilution was calculated as a percentage of the well with no Tregs. *p < 0.05, **p < 0.01, *** p < 0.001 by Mann-Whitney U test.
CD4 T cells were stimulated with PMA/Ionomycin for 4 hours and stained for intracellular FoxP3, IFNg, and IL2 (A). A representative plot of Helios gating is shown (B). (C) FoxP3Lo Helios+ T cells are pregated on Live CD3+CD4+CD127LoCD25+ and graphed as a percentage of total CD4+ T cells. (D) Conventional effector T cells were stimulated with CD3/CD28 dynabeads for 2 days then incubated at indicated ratios with FACS sorted CD45RA+ Tregs for 3 days (RA, CD3+CD4+CD127LoCD25+CD45RA+), CD25Lo Tregs (25Lo, CD3+CD4+CD127LoCD25LoCD45RA-), CD25Hi Tregs (25Hi, CD3+CD4+CD127LoCD25HiCD45RA-); proliferation as measured by Cell trace dye dilution was calculated as a percentage of the well with no Tregs. *p < 0.05, **p < 0.01, *** p < 0.001 by Mann-Whitney U test.
To cite this abstract in AMA style:
Yockey L, Doyle I, Guy T, Trivedi D, Gadiraju C, Bonaso F, Akaa J, Puri A, Wallace Z, Katz G, Montesi S, Stone J, Castelino F, Pillai S, Luster A, Mahajan V, Perugino C. FoxP3Lo CD4+ T cells are functionally suppressive and expanded in the immune-mediated fibrotic diseases IgG4-related disease and systemic sclerosis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/foxp3lo-cd4-t-cells-are-functionally-suppressive-and-expanded-in-the-immune-mediated-fibrotic-diseases-igg4-related-disease-and-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/foxp3lo-cd4-t-cells-are-functionally-suppressive-and-expanded-in-the-immune-mediated-fibrotic-diseases-igg4-related-disease-and-systemic-sclerosis/
