Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Lupus nephritis (LN) is one of the most common complications of systemic lupus erythematosus (SLE), with high morbidity and mortality. Despite a better understanding of the pathogenesis of LN, treatment progress is still insufficient. Dysregulation of cell death and deficient clearance of dying cells lead to the production of autoantigens, induction of autoantibodies, deposition of circulating immune complexes, and other abnormal immune responses in SLE and LN patients. Ferroptosis, a recently discovered PCD, has hardly been reported in detail in LN. Thus, the purpose of this study was to investigate the role of ferroptosis and its associated metabolic pathways in the pathogenesis of LN.
Methods: Transcriptome datasets containing LN and Living donors was obtained from GEO database, and gene sets associated with different PCD modes and different metabolic pathways was extracted from the signaling pathway database or literature search. Composite gene expression scores were calculated by averaging z-scored transformed log2 expressed genes within the pathway. Immunohistochemistry and immunofluorescence were used to detect the expression of 4-HNE and CD163 in LN.
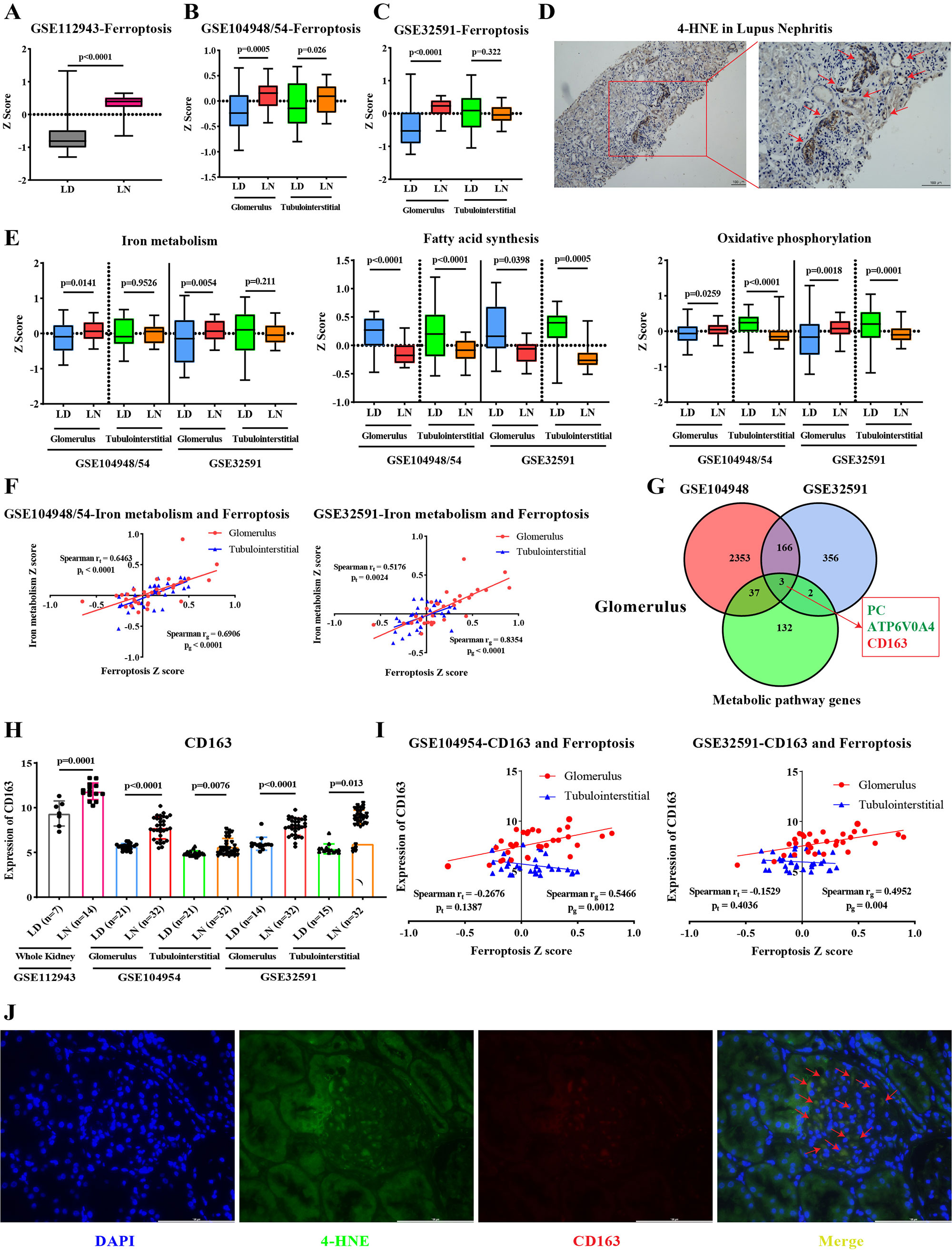
Results: We found that ferroptosis was significantly increased in LN, especially in the glomeruli, while ferroptosis of tubulointerstitial compartment was different in two datasets (Figure 1A, B, C). By immunohistochemistry, we found that the expression of 4-HNE (key medium for ferroptosis) was significantly increased in both glomerulus and tubules (Figure 1D). Next, we explored changes in LN of eight metabolic pathways associated with ferroptosis. In the glomerular compartment, iron metabolism and oxidative phosphorylation pathways were significantly enhanced, while fatty acid synthesis pathway was significantly reduced. In the tubulointerstitial compartment, fatty acid synthesis and oxidative phosphorylation pathways were significantly reduced (Figure 1E). Only iron metabolism and ferroptosis were positively correlated in glomerulus and tubules (Figure 1F). By intersecting differentially expressed genes and metabolism-related genes, we finally obtained one up-regulated gene (CD163) and two down-regulated genes (PC and ATP6V0A4) in glomerulus (Figure 1G, H). The expression of CD163 in glomerulus was positively correlated with ferroptosis (Figure 1I). Meanwhile, immunofluorescence showed that CD163 and 4-HNE co-located in the glomerulus (Figure 1J).
Conclusion: In conclusion, this study reveals that different PCD modes, especially ferroptosis, markedly elevated in LN. In addition, we provided the first in-depth analysis of the changes of different ferroptosis-related metabolic pathways in LN and found that enhanced iron metabolism pathway may be the most important factor in ferroptosis of glomerular. Finally, we identified an upregulated iron metabolism gene, CD163, that was significantly associated with ferroptosis. Due to the lack of studies on the mechanism of CD163+M2 macrophages in LN, we believe that ferroptosis is a very novel and promising option and direction.
To cite this abstract in AMA style:
Cheng Q, Wu H, Du Y. Ferroptosis of CD163+ Tissue Infiltrating Macrophages in Lupus Nephritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/ferroptosis-of-cd163-tissue-infiltrating-macrophages-in-lupus-nephritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ferroptosis-of-cd163-tissue-infiltrating-macrophages-in-lupus-nephritis/