Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
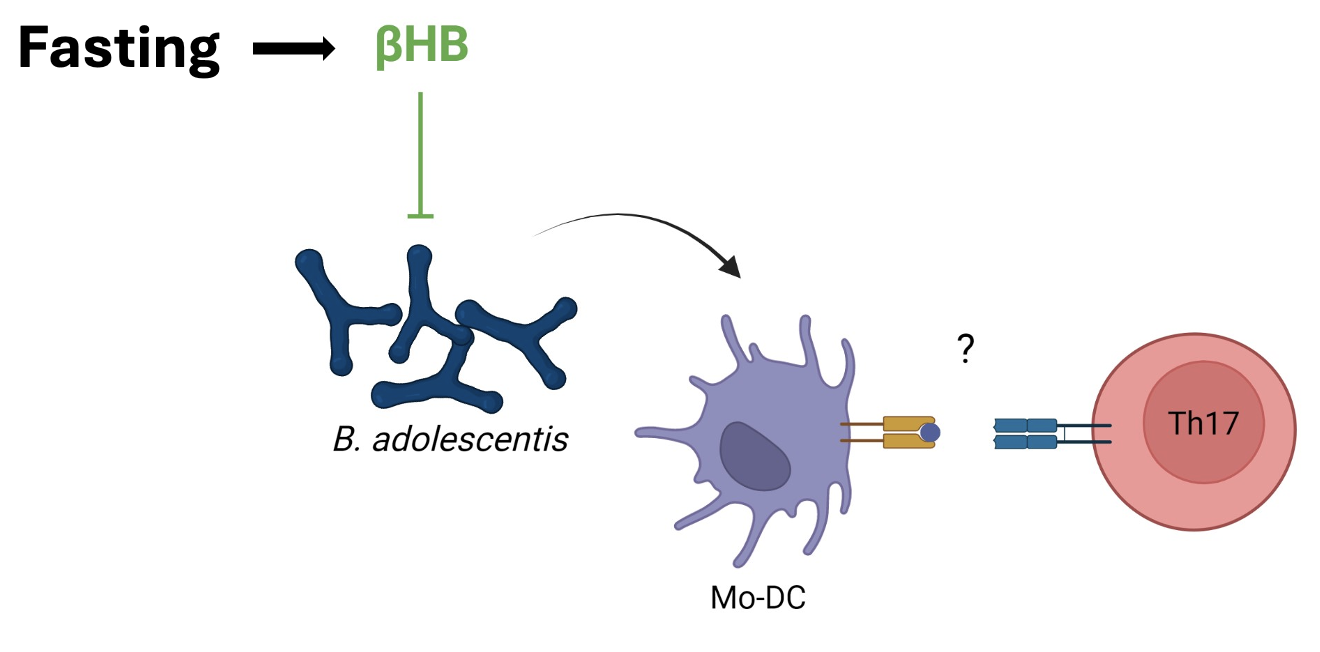
Background/Purpose: The mucosal origins hypothesis suggests rheumatoid arthritis (RA) is triggered at mucosal sites in genetically predisposed hosts1. Animal models support that microbiota‐induced Th17 cells are involved in the pathogenesis of RA2 and other rheumatic diseases3,4. A ketogenic diet in patients with metabolic disease was shown to decrease gut Th17 cells in mice by reducing bifidobacteria via ketone bodies5. However, which host-microbiota interactions are involved in fasting of patients with RA, that improves disease activity clinically6, are unknown. We set out to characterize the gut microbial communities, metabolites, and host-microbiota interactions with a focus on Th17 in a cohort of patients with RA who underwent fasting.
Methods: Stool and serum samples were obtained from a subset of patients with RA enrolled in the NutriFast study6. 10 patients fasting (~300 Kcal) for 7 days and 8 patients under an anti-inflammatory diet were analyzed. Fecal microbiota composition was defined by full-length 16S rDNA sequencing and quantitative PCR. A cytokine-bead array assay was used to measure serum cytokines. Beta-hydroxybutyrate (BHB) was determined via dried blood spot metabolomics. A heat-killed candidate bacterium was co-cultured with PBMCs from healthy individuals to determine human Th17 induction in vitro (n=8). Wilcoxon and Whitney tests were used to compare baseline vs intervention and dietary groups. Linear mixed models were used to evaluate differences in IL-17 levels over time between two groups.
Results: One week of fasting significantly reduced the HAQ score (p = 0.008) and circulating lymphocyte counts (p=0.008) of patients with RA, while increasing ketone bodies (BHB; p = 0.008) compared to an anti-inflammatory diet. The gut microbiota composition was also different between groups. In particular, Bifidobacterium adolescentis (BA) was the most strongly reduced species after seven days of fasting compared to the control group (p= 0.014; LDA score = 5.51), which was confirmed by a species-specific qPCR analysis (p = 0.031). BA+ RA patients, independently of the dietary regimens, displayed higher serum levels of IL-17 than patients not colonized with this species (p=0.037). In vitro studies on human PBMCs revealed that BA induces IL-17+/IFNg+ Th17 cells (p=0.008).
Conclusion: A 7-day fasting intervention in patients with RA altered the gut microbiota compared to an anti-inflammatory diet. Fasting decreased BA, a species which was able to induce pathogenic Th17 cells in vitro. BA was previously shown to induce intestinal Th17 in mice5, exacerbate experimental arthritis7, and disrupt epithelial barrier function8. Altogether, our results put forth a diet-sensitive candidate pathobiont in human RA and delineate a host-microbiota interaction involved in the beneficial effects of fasting.
1. Holers et al., 2018 Nat Rev Rheumatol
2. Evans-Marin et al., 2018 Arthritis Rheumatol
3. Manfredo Vieira et al., 2018 Science
4. Gronke et al., 2023 bioRxiv
5. Ang et al., 2020 Cell
6. Hartmann et al, 2022 Front Nutr
7. Tan et al, 2016 PNAS
8. Bootz-Maoz et al., 2022 Cell Reports
To cite this abstract in AMA style:
Pereira M, Stuhlträger K, Scherff N, Rajput Khokhar A, Redanz S, Ebid H, Hansen B, C. Lacny C, Löschberger U, Bletz S, G. Schneider J, Wilmes P, S. Kessler C, Michalsen A, Mellmann A, Kriegel M. Fasting Reduces an IL-17+/IFNg+ T Helper Cell-inducing Gut Pathobiont in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/fasting-reduces-an-il-17-ifng-t-helper-cell-inducing-gut-pathobiont-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/fasting-reduces-an-il-17-ifng-t-helper-cell-inducing-gut-pathobiont-in-patients-with-rheumatoid-arthritis/