Session Information
Date: Tuesday, October 28, 2025
Title: Abstracts: Spondyloarthritis Including Psoriatic Arthritis – Basic Science (1752–1757)
Session Type: Abstract Session
Session Time: 11:00AM-11:15AM
Background/Purpose: Exosomes are small extracellular vesicles carrying surface molecules and molecular cargo, including microRNAs (miRNAs), that mediate intercellular communication. Both the exosomal cargo and surface markers are increasingly recognized as key players in immune modulation and potential biomarkers in autoimmune diseases. In ankylosing spondylitis (AS), their role remains underexplored. We conducted an integrated investigation of exosomal surface markers, miRNA profiles, and their functional impact on regulatory T cells (Tregs) and purinergic signaling, aiming to identify new diagnostic and therapeutic avenues.
Methods: Plasma-derived exosomes were isolated from AS patients and healthy controls (HC). Exosomal surface markers were analyzed using flow cytometry, and miRNA sequencing was performed to profile cargo signatures. Functional effects were assessed in CD4+ T cells and Tregs by evaluating FOXP3+IRF4+ and FOXP3+IRF4+CTLA4+ subsets, proliferation assays, and CD39/CD73 enzymatic activity. DNA methylation of FOXP3, IL2R, and IRF4 was analyzed in four groups: HC Tregs, AS Tregs, HC Tregs exposed to AS exosomes, and CD4+CD25− controls. Gene expression profiling in sorted Tregs was used to assess key immune regulatory and apoptotic genes.
Results: Gene expression profiling of sorted Tregs from AS patients revealed downregulation of IKZF2/Helios, TNFα, CD95/FAS, BCLXL, and CD45RO, alongside upregulation of IGF1R, indicating disrupted transcriptional networks important for Treg survival and stability. DNA methylation analysis demonstrated hypermethylation of FOXP3, IL2R, and IRF4 in AS Tregs, with similar patterns observed in HC Tregs exposed to AS exosomes, highlighting the epigenetic reprogramming potential of exosomes. miRNA sequencing of AS plasma-derived exosomes identified upregulation of miR-30c-5p, a regulator targeting IRF4, directly linking exosomal cargo to impaired Treg function. Functionally, AS exosomes carrying miR-30c-5p led to decreased proliferation of CD4+ T cells and a marked reduction in FOXP3+IRF4+ and FOXP3+IRF4+CTLA4+ Treg subsets. In parallel, AS exosomes induced reduced CD39/CD73 surface expression and impaired ATPase activity, indicating defective extracellular adenosine production and weakened immunosuppressive signaling. Collectively, these results demonstrate that AS exosomes orchestrate immune dysregulation through parallel effects on Treg stability and purinergic signaling.
Conclusion: Our integrated study demonstrates that AS plasma-derived exosomes drive immune dysregulation through miRNA-mediated gene regulation, epigenetic remodeling, and impaired purinergic signaling, culminating in Treg instability and loss of immune tolerance. These findings highlight exosomal signatures as promising biomarkers and therapeutic targets to restore immune balance in AS.
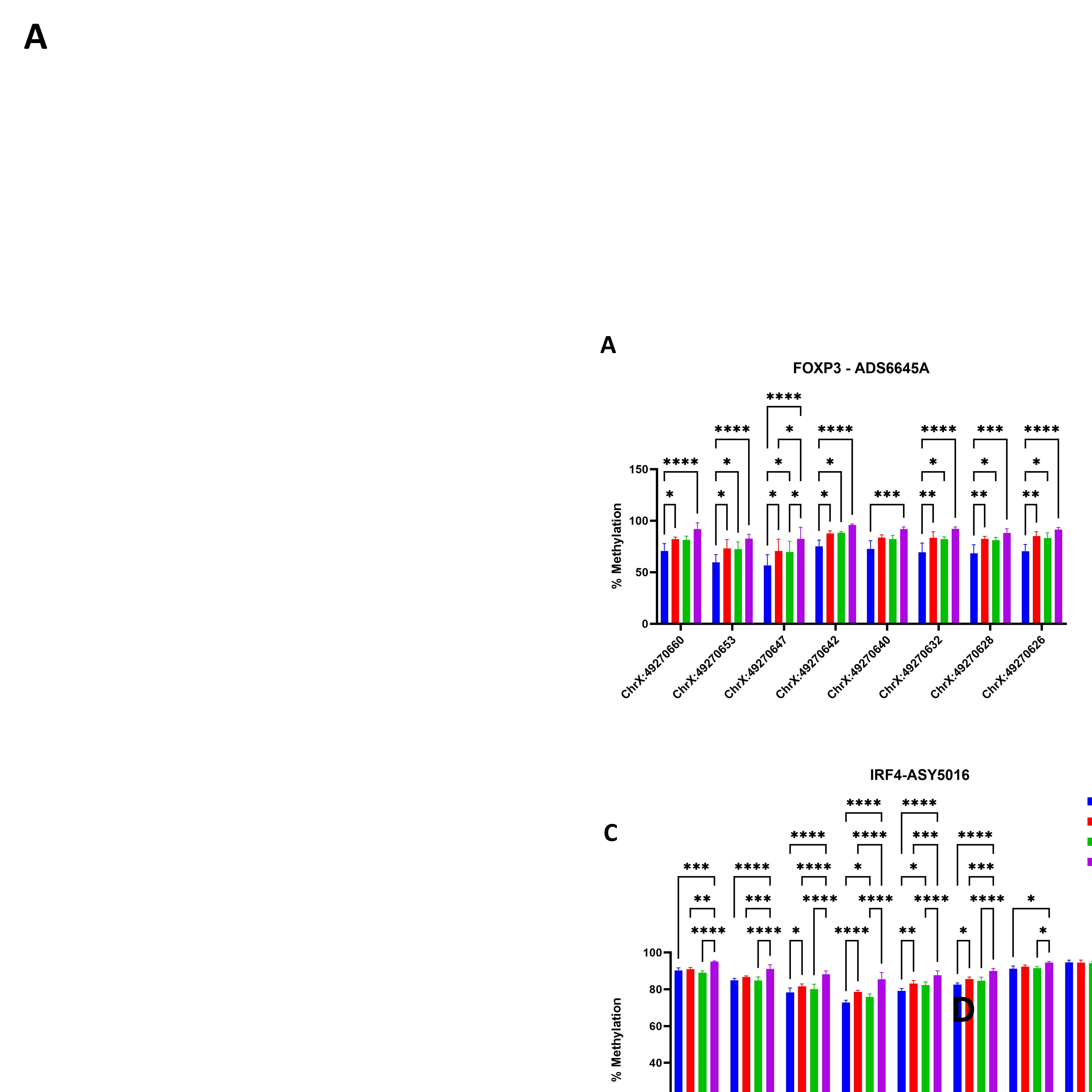 Figure 1- Exosome Isolation and Validation :(a) Exosome characteristics with morphology, size and markers. Exosomes display a round-shaped morphology in negative transmission electron microscopy (TEM). It is at 22 000× magnification, demonstrating the density of isolated exosomes in plasma (b) and are bilayer and spherical by cryo-TEM imaging. (c) Exosome sizes can be determined using nanoparticle tracking. Nanoparticle tracking analysis was used to examine the exosome size and concentration, and the results showed that the exosomes had an average size of 147 nm. (d) Expression of the exosomal markers CD63 and CD9 was confirmed with western blot.
Figure 1- Exosome Isolation and Validation :(a) Exosome characteristics with morphology, size and markers. Exosomes display a round-shaped morphology in negative transmission electron microscopy (TEM). It is at 22 000× magnification, demonstrating the density of isolated exosomes in plasma (b) and are bilayer and spherical by cryo-TEM imaging. (c) Exosome sizes can be determined using nanoparticle tracking. Nanoparticle tracking analysis was used to examine the exosome size and concentration, and the results showed that the exosomes had an average size of 147 nm. (d) Expression of the exosomal markers CD63 and CD9 was confirmed with western blot.
.jpg) Figure 2: Immune Dysregulation and Exosome-Mediated Adenosine Modulation in axial spondyloarthritis (AS)
Figure 2: Immune Dysregulation and Exosome-Mediated Adenosine Modulation in axial spondyloarthritis (AS)
(A) Immune Dysregulation in AS: The enthesitis microenvironment (EME) in AS is characterized by cellular interactions that drive inflammation and pathological bone remodeling. Panel A depicts cellular interactions where IL-23 stimulates Th17 cells to produce IL-17 and TNF-α, recruiting macrophages and neutrophils to the entheses. The cytokine release amplifies inflammation, leading to tissue damage and fibrosis. Panel B illustrates the roles of fibroblasts, macrophages, and T cells in the dysregulated cytokine signaling cascade. IL-17 and TNF-α receptor activation stimulate fibroblasts to release RANKL and MMP, driving osteoclast activity and disrupting bone homeostasis. Concurrently, osteoblast activation leads to pathological bone formation (syndesmophytes), highlighting the imbalance in bone remodeling. Panel C demonstrates genetic dysregulation in AS, with HLA-B27 misfolding inducing ER stress, promoting IL-23 production, and driving Th17-mediated inflammation. Panel D emphasizes cytokine-driven bone remodeling, showing osteoclast-mediated bone resorption and osteoblast-driven syndesmophyte formation under persistent inflammation.
(B) Exosome-Mediated Adenosine Modulation of Inflammation and Bone Dynamics in AS: Exosomes released from immune and stromal cells deliver enzymes (CD39/CD73) and miRNAs (e.g., miR-146a, miR-29b) to modulate immune responses and bone remodeling in AS. CD39/CD73-mediated adenosine production suppresses osteoclast activity and enhances osteoblast activity via A2A receptor signaling. While adenosine reduces RANKL and MMP expression in fibroblasts and promotes M2 macrophage polarization, the reduced CD39/CD73 expression observed in AS limits adenosine availability, impairing its anti-inflammatory and bone-regulating effects. The balance between osteoclast-mediated bone erosion and osteoblast-mediated syndesmophyte formation is critical to AS pathology. Arrows indicate cellular interactions and regulatory pathways modulated by adenosine and exosomal components.
Figure 3. DNA Methylation Analysis. A: Hypermethylation of FOXP3 in AS Treg cells compared to HC. B: Hypermethylation of IL2R in AS Treg cells, contrasting with the typical demethylation observed in Tregs. C & D: Hypermethylation of IRF4 in Treg cells may impair their function in AS. This alteration may impact Treg function. HC Treg cells exposed to AS exosomes exhibited similar methylation patterns to AS Treg cells in all figures, highlighting the role of AS exosomes in Treg dysfunction.
To cite this abstract in AMA style:
Tavasolian F, lively s, Pastrello c, Lim M, Pacheco A, Qaiyum Z, Tang M, Baskurt Z, Jurisica I, Kapoor M, Inman R. Exosomal Cargo and Surface Markers: Informative Signals in Axial Spondyloarthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/exosomal-cargo-and-surface-markers-informative-signals-in-axial-spondyloarthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/exosomal-cargo-and-surface-markers-informative-signals-in-axial-spondyloarthritis/

.jpg)