Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Idiopathic retroperitoneal fibrosis (RPF) / chronic periaortitis (CP) is an uncommon condition characterized by a fibroinflammatory periarterial soft tissue thickening around the infra-renal abdominal aorta, often with extension into the common iliac arteries. The periaortic inflammation can result in ureteral obstruction causing hydronephrosis and renal compromise, but narrowing or occlusion of the inferior vena cava (IVC) and iliac veins can also occur producing marked lower extremity edema. Limited data is available describing iliocaval stenting in RPF/CP and consequently information on long-term patency is lacking.
Methods: All patients with RPF/CP diagnosed and managed at our institution from January 1, 1998 through December 31, 2019 were identified through direct medical chart review. Patients with at least one venous stent in the IVC, iliac, or femoral vein with follow-up were included in this study. Rate of patency was estimated using Kaplan-Meier methods. Assessment of age, sex, use of prednisone or immunomodulatory therapy at stent placement and type of post-procedural anticoagulation, were evaluated for prediction of venous stent occlusion using Cox proportional hazard models.
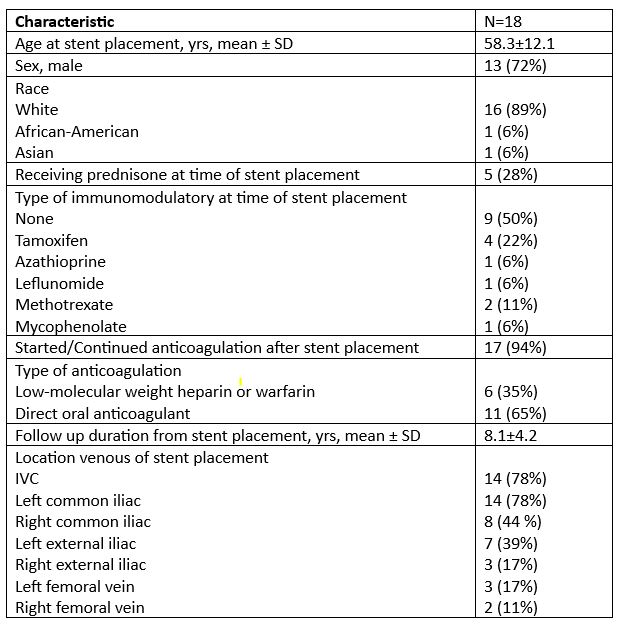
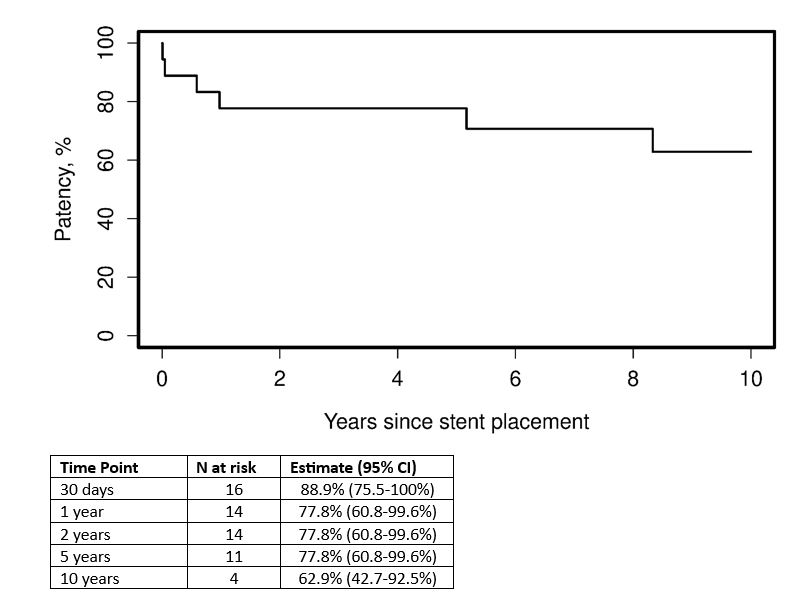
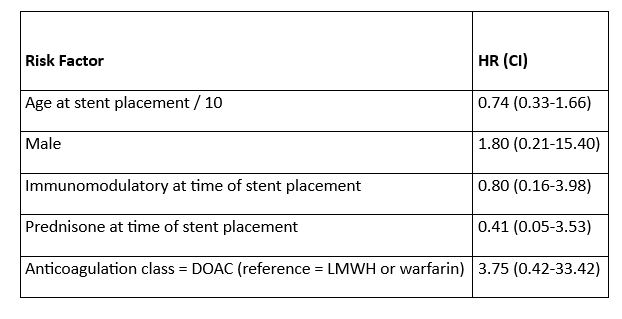
Results: Eighteen patients (13 male, 5 female) were included in this study. Patient characteristics are highlighted in Table 1. Five patients (28%) were on prednisone and nine (50%) were on an immunomodulatory agent at time of stent placement. Only 2 patients had IVC only stent, 4 patients iliac or femoral only stents. The remainder had a combination of IVC plus one or more iliac/femoral stenting (Table 1). Anticoagulation was started or continued in all but one patient following stent placement, the most common class was direct oral anticoagulant (11/17, 65%). Occlusion occurred in two patients within 30 days. Rate of patency was 77.8% [95% CI, 60.8-99.6%] at years 1, 2, and 5 (Figure 1). Neither age, sex, presence of prednisone or presence of immunomodulatory at time of stent placement, nor anticoagulation class was able to predict risk of occlusion (Table 2).
Conclusion: This cohort reports the largest, single-institution cohort evaluating patency of iliocaval/iliofemoral stent placement for non-thrombotic, non-malignant venous obstruction associated with RPF/CP. Occlusion rates within 5 years after stent placement are low. Age, sex, immunotherapy, and anticoagulation did not predict risk of occlusion. Evaluation of larger cohorts of patients are needed to understand which parameters may be associated with an increased risk of occlusion in this unique patient population.
To cite this abstract in AMA style:
Lay R, Achenbach S, Crowson C, Ghaffar U, Burke M, Bjarnason H, Neidert N, Warrington K, Koster M. Evaluation of Long-term Primary Patency of Iliocaval Stenting in Patients with Retroperitoneal Fibrosis/chronic Periaortitis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/evaluation-of-long-term-primary-patency-of-iliocaval-stenting-in-patients-with-retroperitoneal-fibrosis-chronic-periaortitis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-long-term-primary-patency-of-iliocaval-stenting-in-patients-with-retroperitoneal-fibrosis-chronic-periaortitis/