Session Information
Date: Monday, November 14, 2022
Title: Abstracts: RA – Diagnosis, Manifestations, and Outcomes II: Cardiovascular and Other Comorbidities
Session Type: Abstract Session
Session Time: 9:00AM-10:30AM
Background/Purpose: Patients with rheumatoid arthritis (RA) have a higher prevalence of coronary artery disease (CAD) than the general population, which contributes to early mortality. However, CAD screening tools developed in the general population are less effective for estimating CAD risk in RA patients, due to the differing contribution from traditional risk factors and the contribution from disease-specific factors. Understanding of the genetic basis of CAD has improved over recent years and shows promise for improving risk prediction in the form of genetic risk scores (GRS), in particular with the development of the metaGRS approach, which combines multiple GRS. This study hypothesises that the metaGRS approach can improve CAD risk prediction in patients with RA.
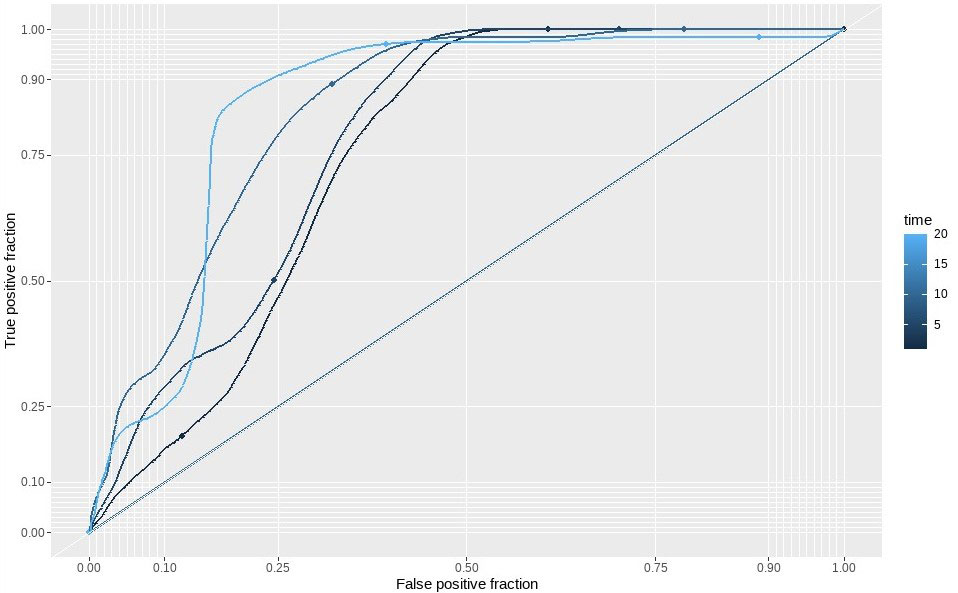
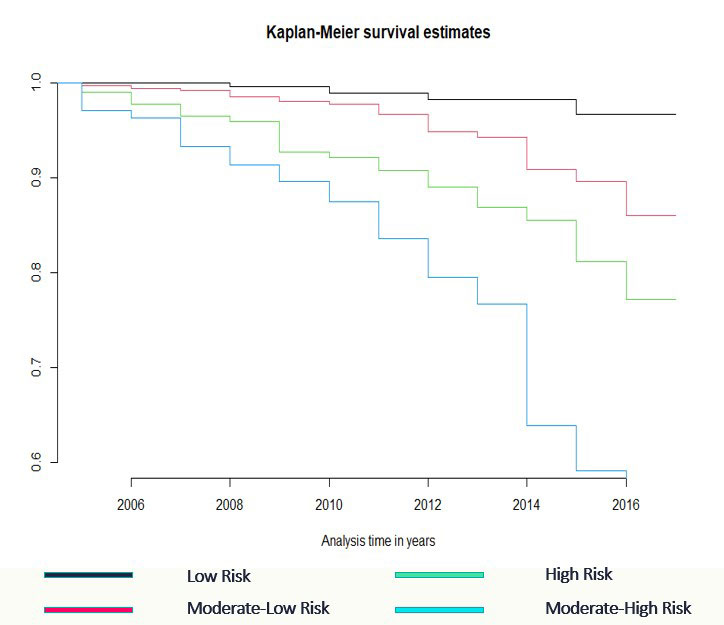
Methods: Patients were recruited from the Norfolk Arthritis Register (NOAR), a detailed community-based longitudinal observational study focused on the cause and outcome of inflammatory polyarthritis, between 1990 to 2017. Analysis was restricted to patients who satisfied the 2010 ACR criteria cumulatively over five years and had detailed clinical history at baseline and follow-up. We developed a prediction model based on traditional risk factors and explored the inclusion of a single CAD GRS (49K SNPs). We also used a meta-analytic approach to calculate a new metaGRS for CAD, using the effect-sizes from three large-scale, genome-wide, and targeted CAD GRS derived from 1,745,179, 6,630,150, and 40,079 SNPs in a subset of patients with available genetic data. Cox proportional hazards models were used to derive risk equations for evaluation of 10-year risk of CAD. We applied multiple imputations with chained equations using the Random Forest algorithm to replace missing values. Measures of calibration and discrimination were determined in the validation cohort of 423 individuals.
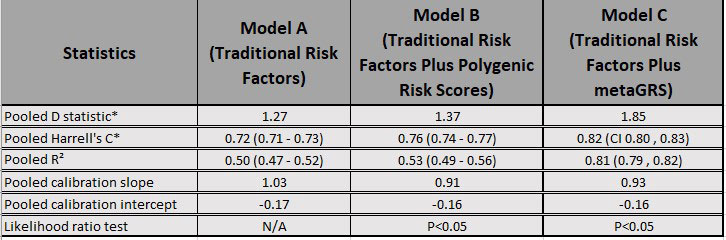
Results: A total of 2123 patients were included in the analysis with 136 incident cases of self-reported CAD (defined as a composite outcome of myocardial infarction, angina, heart attack, arrhythmia, angioplasty, and coronary artery bypass grafting). The model using only traditional risk factors achieved an AUC of 0.72 (95% CI 0.71, 0.73), with a calibration slope of 1.03, and explained approximately 50% (95% CI 47, 52%) of the variance of the outcome. The hazard ratio for age was found to be 1.00 (95% CI 0.99, 1.01) indicating risk remains the same across all age groups. Inclusion of a CAD GRS increased the performance with an AUC of 0.76 (95% CI 0.75, 0.77), explained variance of 53% (95% CI 49, 56%) but with a slightly worse calibration slope of 0.91. Inclusion of a CAD metaGRS improved the AUC to 0.82 (95% CI 0.80, 0.83), explained more of the variance at 81% (95% CI 79, 82%) with a calibration slope of 0.93. A likelihood ratio test indicates that the integrated model is a better fit (p = 0.04).
Conclusion: An integrated risk score, that combines traditional risk factors with a metaGRS, improves CAD prediction in patients with RA. Further research is required to better understand the role of heritable components contributing to CAD risk in RA patients. By refining the underlying GRS, risk prediction may be further improved through this integrated approach.
To cite this abstract in AMA style:
Soomro M, Stadler M, Viatte S, Macgregor A, Verstappen S, Barton A, Bowes J. Evaluating the Use of Genetic Risk Scores as Part of an Integrated Risk Tool for Predicting Coronary Artery Disease in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/evaluating-the-use-of-genetic-risk-scores-as-part-of-an-integrated-risk-tool-for-predicting-coronary-artery-disease-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluating-the-use-of-genetic-risk-scores-as-part-of-an-integrated-risk-tool-for-predicting-coronary-artery-disease-in-patients-with-rheumatoid-arthritis/