Session Information
Date: Sunday, November 8, 2020
Title: Patient Outcomes, Preferences, & Attitudes Poster II: Miscellaneous Rheumatic Diseases
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Juvenile myositis (JM) causes weakness, rashes, pain, and fatigue, thereby impacting health-related quality of life (HRQoL). Patient-Reported Outcomes Measurement Information System (PROMIS®) measures have undergone initial validation in JM, but clinical interpretability is limited. Estimates of clinically important differences (CIDs) have not yet been determined for PROMIS measures in JM. This study estimates CIDs of PROMIS fixed short forms in JM.
Methods: JM patients (5-17 yo) and their parents were recruited at routine clinic visits. Clinical/demographic data were collected. Patients (8-17 yo) and parents (5-17 yo) completed PROMIS pediatric fixed short forms for Anxiety, Depressive Symptoms, Mobility, Upper Extremity Function, Fatigue, and Pain Interference domains. Distribution-based estimates (i.e. 0.5 standard deviation) of CIDs were calculated. Cross-sectional and longitudinal anchor-based estimation of CIDs was performed. Longitudinal anchor-based CID estimates (i.e. change in PROMIS score associated with change in anchor variable) were calculated for patient-parent dyads with data collected at 2 study visits. Anchor variables collected at study visits included: Physician’s Global Assessment of Disease Activity (PGA), muscle enzymes, Disease Activity Score Muscle and Skin Domains (MDAS, SDAS), Childhood Myositis Assessment Scale (CMAS), PedsQL Generic Core Scales (PedsQL-GC) and PedsQL Rheumatology Modules (PedsQL-RM). Anchor variables were grouped into clinically distinct categories based on published score cutoffs. Criteria for reporting anchor-based CID estimates included: 1) Spearman’s correlation ≥0.3; 2) n ≥5 per clinically distinct anchor variable category; 3) effect size (i.e. Cohen’s D) > 0.2. Differences in mean PROMIS scores across adjacent clinically distinct anchor variable categories were reported as CID estimates. Mean standard error of measurement (SEM) for each PROMIS domain served as the lower bound for CID estimates.
Results: Seventy-five patient-parent dyads enrolled, with descriptive data in Table 1. Mean SEM for PROMIS child report and parent report were respectively: Anxiety (4.7; 3.9), Depressive Symptoms (4.7; 4.5), Fatigue (4.6; 3.5), Mobility (5.3; 4.6), Pain Interference (4.4; 3.9), Upper Extremity Function (6.5; 5.7). Distribution-based PROMIS CID estimates ranged 3.4-6.1 and 4.7-6.3 for child report and parent report respectively. Cross-sectional anchor-based CID estimates are shown in Table 2. Longitudinal anchor-based CID estimates are presented in Table 3.
Conclusion: Our CID estimates enhance interpretability of PROMIS measures in JM by linking differences in PROMIS measures with relevant clinical and patient-reported anchors. CIDs can support the design and power calculations for future studies (e.g. clinical trials). Future multicenter studies should enhance precision and generalizability of CID estimates and assess differences in CIDs by disease status (e.g. high vs low disease activity).
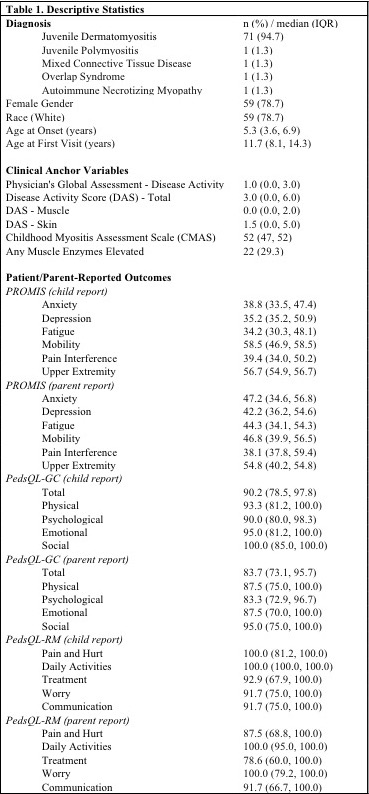 Table 1: Descriptive Statistics
Table 1: Descriptive Statistics
 Table 2: Cross-Sectional Anchor-Based Clinically Important Differences (CIDs)
Table 2: Cross-Sectional Anchor-Based Clinically Important Differences (CIDs)
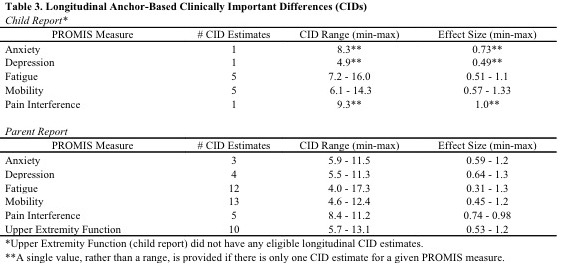 Table 3: Longitudinal Anchor-Based Clinically Important Differences (CIDs)
Table 3: Longitudinal Anchor-Based Clinically Important Differences (CIDs)
To cite this abstract in AMA style:
Wolfe M, Robinson A, Lai J, Coles T, Gray E, Chang R, Cella D, Ardalan K. Estimation of Clinically Important Differences in Patient-Reported Outcomes Measurement Information System (PROMIS) Measures in Juvenile Myositis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/estimation-of-clinically-important-differences-in-patient-reported-outcomes-measurement-information-system-promis-measures-in-juvenile-myositis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/estimation-of-clinically-important-differences-in-patient-reported-outcomes-measurement-information-system-promis-measures-in-juvenile-myositis/
