Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Ankylosing spondylitis (AS) is an immune-related chronic inflammatory disease characterized by inflammatory pain in the lower back and spinal ankylosing, accompanied by immune system disorders, such as increased inflammatory cytokines and changes in the composition of immune cell subsets. The aging process is associated with immune system aging, which has been previously linked to autoimmune diseases. It remained unknown whether aging process was present in AS and played a pathogenic role. The aim of this study was to investigate the possible mechanism of involvement in AS pathogenesis.
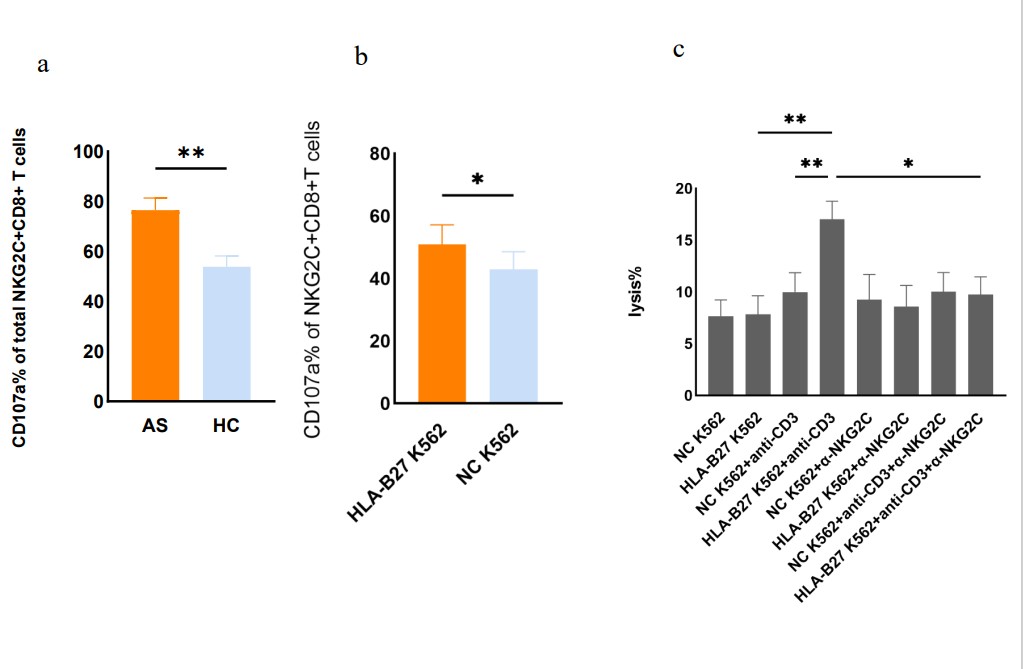
Methods: Peripheral blood mononuclear cell (PBMC) single-cell sequencing and peripheral blood multi-dimensional flow cytometry were performed in AS patients and healthy controls to identify the different cell populations in AS patients. The phenotype and function of NKG2C+CD8+T cells were detected by flow cytometry. Cytotoxicity was determined by the expression of CD107a. HLA-B27 over-expressed K562 cell line was constructed by lentivirus transfection, the effect of HLA-B27 on the function of NKG2C+CD8+T cells was verified by in vitro cell co-culture experiment. Immunofluorescence staining was used to determine the infiltration of Ly49h+ cells in the disease site of collagen-induced arthritis(CIA) mice.
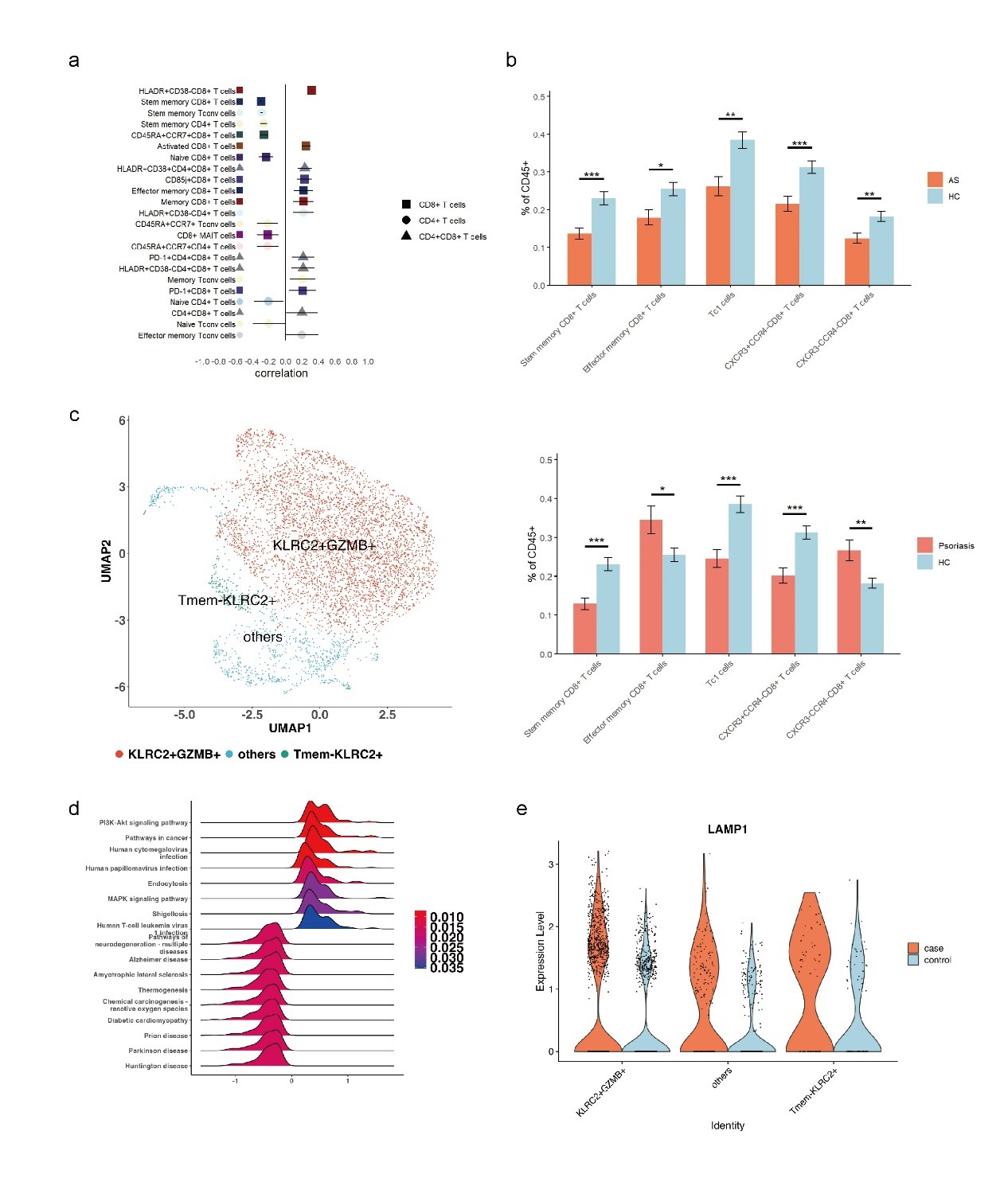
Results: Through a comprehensive analysis peripheral blood multi-dimensional flow panel, we found that the distribution of subsets of CD8+T cells were significantly associated with aging and was abnormal in both AS and psoriasis (Ps) patients. Based on scRNA-seq, we identified the PI3K-Akt signaling pathway and endocytosis pathways of NKG2C+CD8+T cell subset – a well-known aging associated cell type – were activated in AS (Figure 1). Furthermore, we found that the cytotoxicity of this subset was significantly higher in AS with in vitro stimuli. Importantly, we found that the presenting cells with the HLA-B27 molecule led to increased cytotoxicity of this subset, which was diminished with the inhibition of NKG2C.This indicates that NKG2C+CD8+T cells are important effector cells for HLA-B27, and inhibition of NKG2C represents a novel potential target (Figure 2). Additionally, these results were consistent with findings in HLA-B27 transgenic mice, where Ly49h+ cells, a homologous receptor with a similar function to human NKG2C, infiltrated the disease site of CIA mice.
Conclusion: The NKG2C+CD8+T cell subgroup – an aging associated subset, acquires cytotoxic potential with HLA-B27 stimulation. Inhibition of NKG2C represents a novel potential target for AS treatment.
To cite this abstract in AMA style:
Tang K, Ma X, Gao J, Qian F, Zhu Q, Wang J, Liu J. Enhanced Cytotoxicity of Aging Associated NKG2C+ CD8+ T Cells in Ankylosing Spondylitis via HLA-B27 [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/enhanced-cytotoxicity-of-aging-associated-nkg2c-cd8-t-cells-in-ankylosing-spondylitis-via-hla-b27/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/enhanced-cytotoxicity-of-aging-associated-nkg2c-cd8-t-cells-in-ankylosing-spondylitis-via-hla-b27/