Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Neutrophils and neutrophil extracellular traps (NETs) contribute to the vascular complications of multiple diseases, but their role in systemic sclerosis (SSc) is understudied. We sought to test the hypotheses that NETs are implicated in SSc vasculopathy, and that treatment with prostacyclin analogs may ameliorate SSc vasculopathy not only through vasodilation but also by inhibiting NET release.
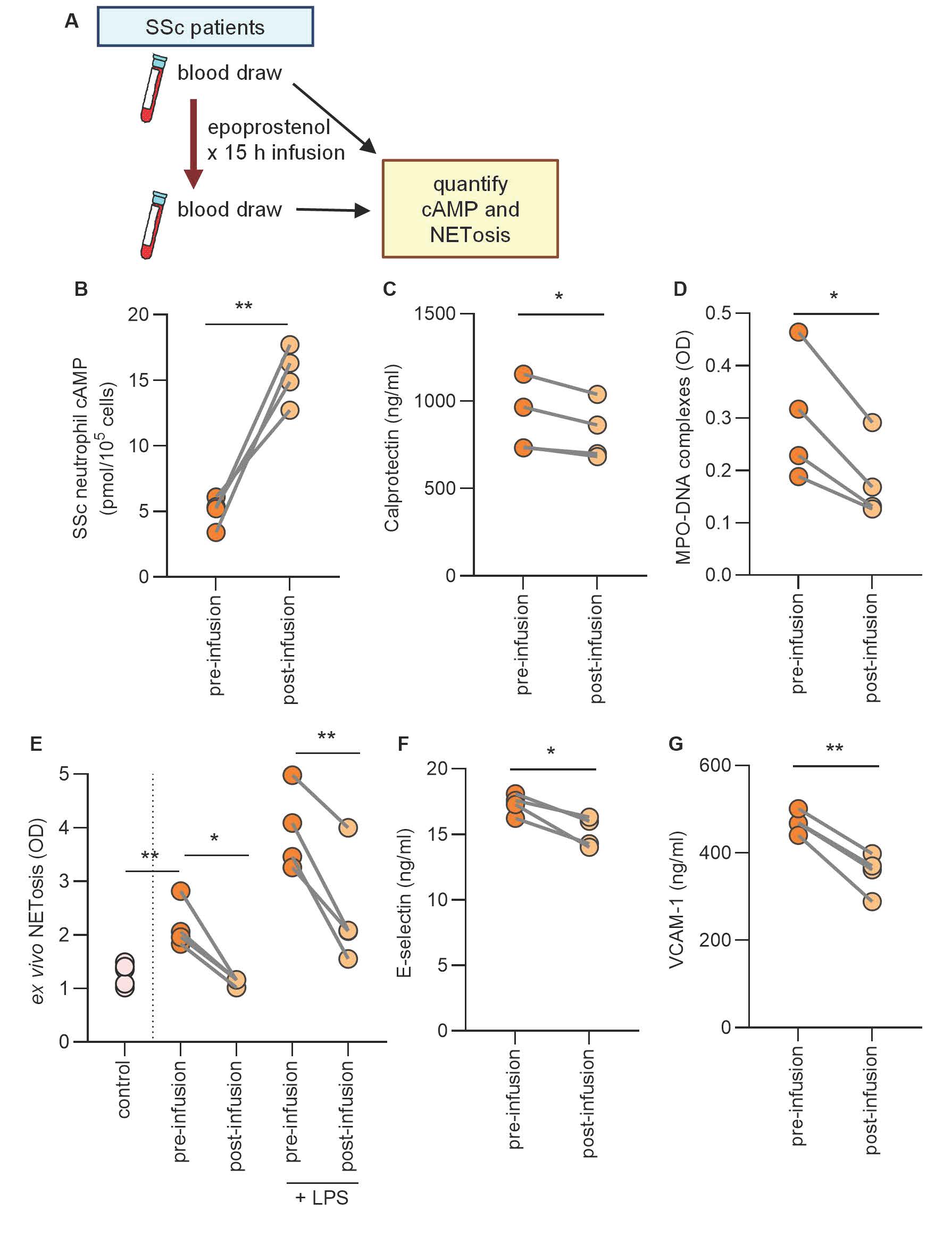
Methods: Plasma from 125 patients with SSc (87 diffuse cutaneous SSc and 38 limited cutaneous SSc) was collected, and vascular complications such as digital ulcers, pulmonary artery hypertension, and scleroderma renal crisis were recorded. We compared SSc patients with known vascular complications (n=54) to a cohort matched for age, sex, and disease duration but without vascular complications (n=71). To determine the impact of prostacyclin analogs, we characterized four SSc patients with digital ischemia admitted for epoprostenol infusion (0.45 mg administered over 30 hours). Blood was collected the week before the infusion and 15 hours after the start of the infusion. Neutrophils were isolated to determine the impact of epoprostenol on neutrophil properties including NET release.
Results: We found that neutrophil activation and NETs were increased in patients with SSc-associated vascular complications compared to matched patients without vascular complications: calprotectin (mean 1213 ng/ml versus 975 ng/ml, P=0.004) and MPO-DNA complexes (mean OD 0.36 versus 0.28, P=0.003). As expected, the association of neutrophil activation and NETs with SSc vascular complications was more evident in active vascular disease (digital ulcers and pulmonary hypertension) than in historical vascular disease (scleroderma renal crisis). We also found that neutrophil activation and NETs were positively correlated with circulating markers of vascular injury: soluble E-selectin (R=0.209, P=0.021 for calprotectin versus R=0.245, P=0.006 for NETs) and VCAM-1 (R=0.312, P=0.005 for calprotectin versus R=0.202, P=0.025 for NETs). Treatment of SSc patients experiencing active digital ischemia with a synthetic prostacyclin analog (Figure 1) boosted neutrophil cyclic AMP content (P=0.005), which was associated with the blunting of ex vivo NET release (P=0.005), circulating calprotectin (P=0.028), and circulating MPO-DNA complexes (P=0.017).
Conclusion: Our study demonstrates an association between NETs and vascular complications in SSc that is more evident in the setting of active disease. We also identified the potential for synthetic prostacyclin analogs to reduce neutrophil activation and NET release in SSc patients.
To cite this abstract in AMA style:
Kortam N, Liang W, Shiple C, Huang S, Gedert R, St. Clair J, Sarosh C, Foster C, Tsou E, Varga J, Knight J, Khanna D, Ali R. Elevated Neutrophil Extracellular Traps in Systemic Sclerosis-associated Vasculopathy and Suppression by a Synthetic Prostacyclin Analog [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/elevated-neutrophil-extracellular-traps-in-systemic-sclerosis-associated-vasculopathy-and-suppression-by-a-synthetic-prostacyclin-analog/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/elevated-neutrophil-extracellular-traps-in-systemic-sclerosis-associated-vasculopathy-and-suppression-by-a-synthetic-prostacyclin-analog/