Session Information
Date: Monday, November 18, 2024
Title: Plenary III
Session Type: Plenary Session
Session Time: 9:00AM-10:30AM
Background/Purpose: Sjögren’s disease (SjD) is a chronic, systemic autoimmune disease characterized by the presence of specific autoantibodies (AAb) and lymphocytic infiltration of exocrine glandular tissues. Nipocalimab, an anti-neonatal Fc receptor (FcRn) mAb, reduces circulating IgG including AAb, by selectively blocking IgG–FcRn interactions. Here, we evaluated the efficacy and safety of nipocalimab in patients (pts) with primary SjD.
Methods: A phase 2 study (DAHLIAS; NCT04968912) included adults (18-75 years) with moderately-to-severely active primary SjD (total Clinical European League Against Rheumatism Sjögren’s Syndrome Disease Activity Index [clinESSDAI] ≥6) who were seropositive for anti-Ro60/-Ro52 IgG antibodies. Randomized pts (1:1:1) received intravenous (IV) nipocalimab 5 or 15 mg/kg, or placebo (PBO) every 2 weeks (W) through W22 and protocol-permitted background standard of care. The primary endpoint of change from baseline (BL) in clinESSDAI score at W24 was chosen to avoid mechanistic bias with IgG levels. Safety was assessed through W30.
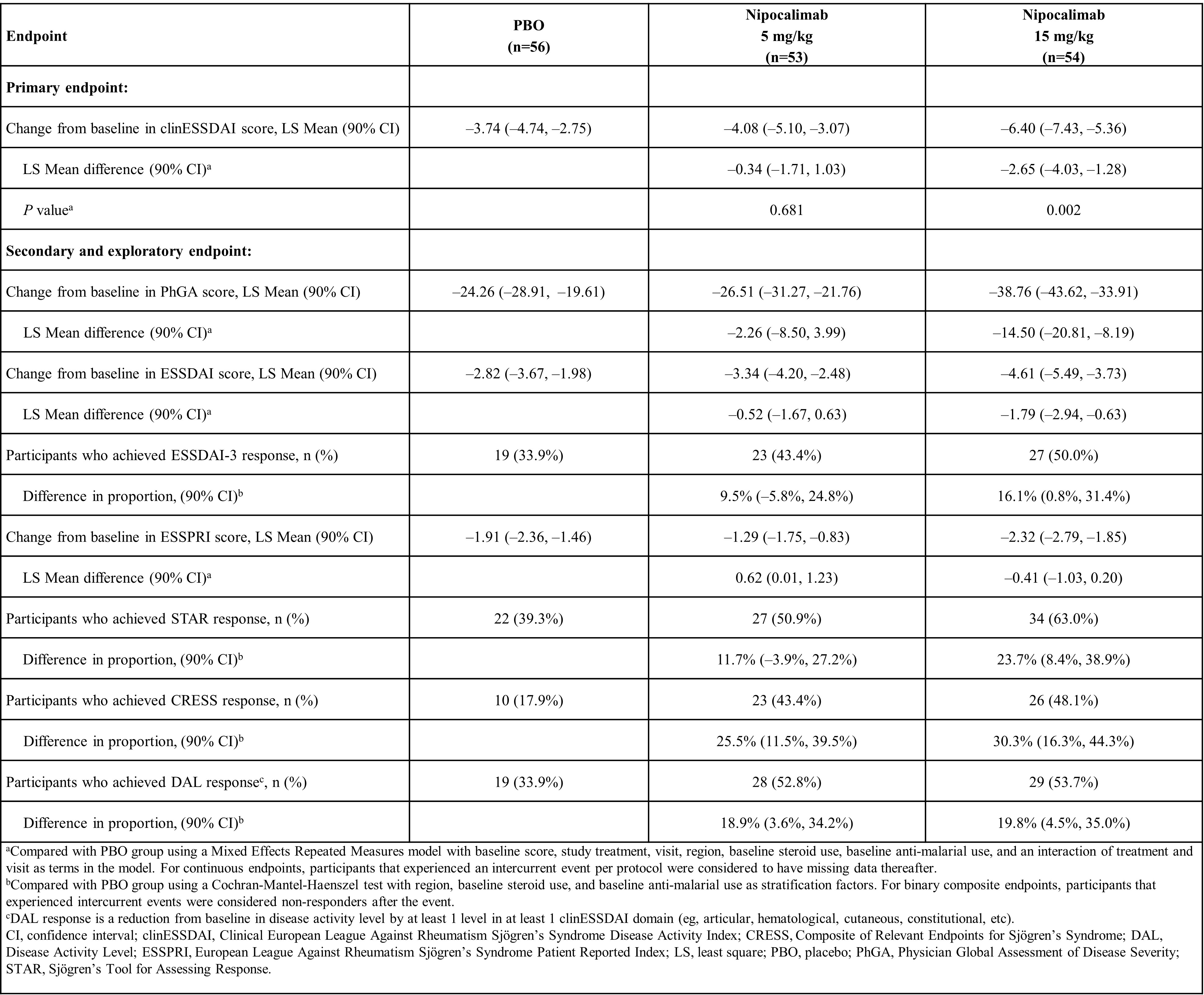
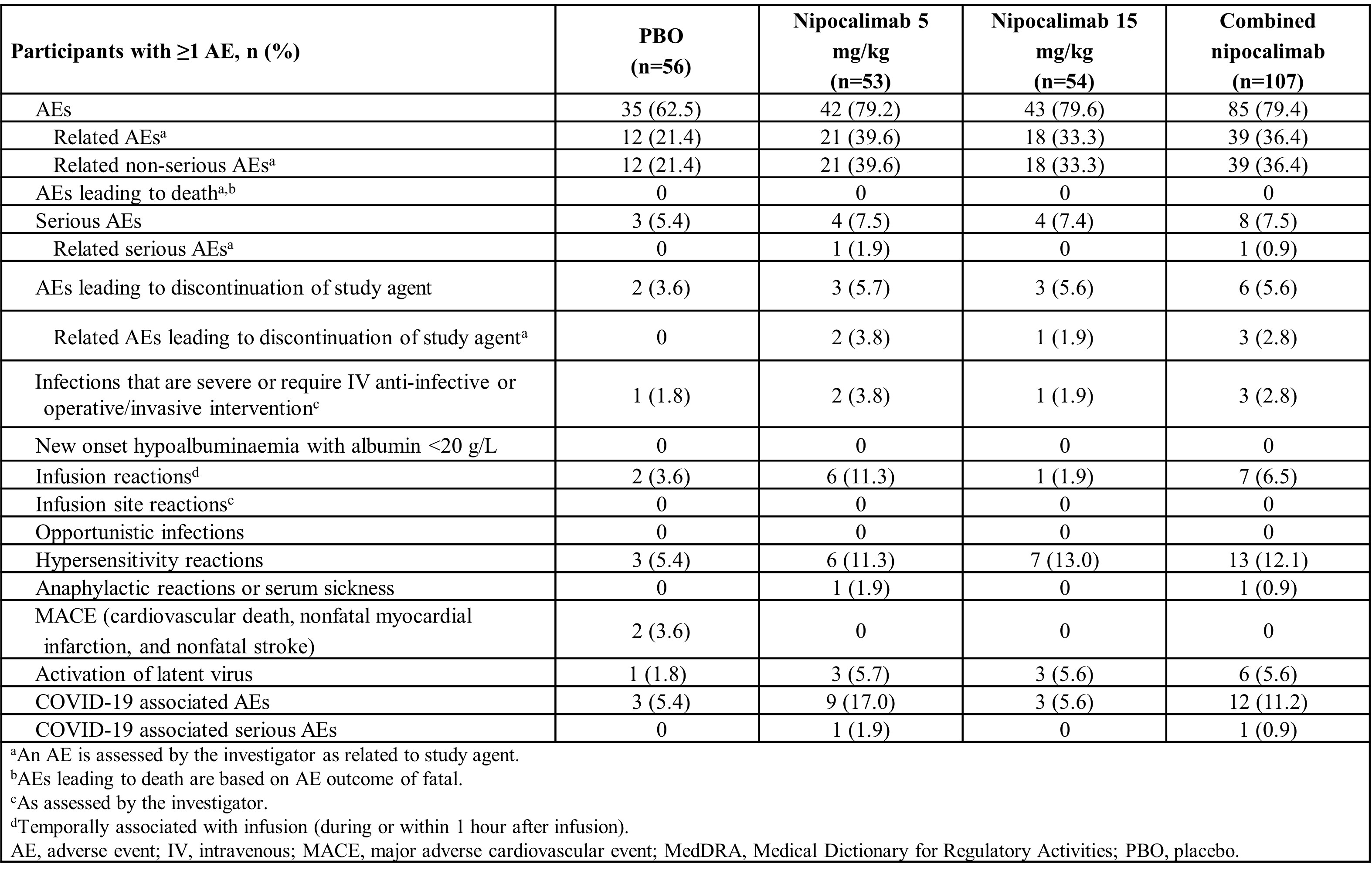
Results: In total, 163 pts were enrolled (nipocalimab: 5 mg/kg, n=53; 15 mg/kg, n=54; PBO, n=56). BL characteristics were comparable between groups. The nipocalimab 15 mg/kg group met the primary endpoint versus (vs) PBO (least squares mean difference for 15 mg/kg: –2.65; 90% CI, –4.03, –1.28; p=0.002; for 5 mg/kg: –0.34; 90% CI, –1.71, 1.03; p=0.681; Table 1). Similar improvements in the 15 mg/kg nipocalimab group vs PBO were observed in most secondary/exploratory endpoints (Table 1), including changes from BL at W24 in Physician Global Assessment of Disease Severity and ESSDAI score, treatment response according to Sjögren’s Tool for Assessing Response, Composite of Relevant Endpoints for Sjogren’s Syndrome, and disease activity level response (decrease in ≥1 clinESSDAI domain), among others. Pt-reported outcome measures numerically improved in the 15 mg/kg nipocalimab group vs PBO. Efficacy outcomes in the 5 mg/kg nipocalimab group were generally similar to PBO. Serious adverse events were reported in 7.5%, 7.4%, and 5.4% of participants with nipocalimab 5 mg/kg, 15 mg/kg, and PBO, respectively (Table 2). Significant nipocalimab dose-dependent reductions in IgG and AAb were observed. Severe infections/infections requiring IV anti-infectives occurred in 3.8%, 1.9%, and 1.8% of patients, respectively, without a clear correlation with IgG nadir; none were related to study treatment. At W24, changes from BL in albumin, low-density lipoprotein cholesterol, and total cholesterol were reported at –6.9%, 6.6%, and 8.3%, respectively. No opportunistic infections/severe hypoalbuminemia/deaths were reported.
Conclusion: DAHLIAS, the first study of a FcRn blocker in SjD, showed that nipocalimab led to significant improvement vs PBO in clinESSDAI and similar trends in other key efficacy endpoints at W24, and is well-tolerated. These results demonstrate the mechanistic relevance of FcRn inhibition in SjD, indicating the potential pathogenicity of IgG AAb. These findings establish proof of concept for nipocalimab in SjD and support further evaluation in pts with moderate-to-severe AAb-positive SjD.
To cite this abstract in AMA style:
Gottenberg J, Sivils K, Campbell K, Idokogi J, Lo K, Liva S, Shelton J, Dhatt H, Hubbard J, Noaiseh G. Efficacy and Safety of Nipocalimab, an Anti-FcRn Monoclonal Antibody, in Primary Sjogren’s Disease: Results from a Phase 2, Multicenter, Randomized, Placebo-Controlled, Double-Blind Study (DAHLIAS) [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-nipocalimab-an-anti-fcrn-monoclonal-antibody-in-primary-sjogrens-disease-results-from-a-phase-2-multicenter-randomized-placebo-controlled-double-blind-study-dah/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-nipocalimab-an-anti-fcrn-monoclonal-antibody-in-primary-sjogrens-disease-results-from-a-phase-2-multicenter-randomized-placebo-controlled-double-blind-study-dah/