Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Immune checkpoint inhibitors (ICI) are a novel cancer therapeutic that have had a dramatic impact on treating advanced malignancies by enhancing the anti-tumor T cell response. However, the ongoing activation of T cells also cause autoimmune side effects, termed immune-related adverse events (irAE). Autoantibodies are present in many patients with irAE and, in lung cancer patients, autoantibodies have been associated with survival (Toi et al. JAMA Onc 2019). Given the similarity of some irAE to de novo rheumatic diseases, autoantibodies could also serve as biomarkers of organ specific irAE. In this study we asked whether prototypical rheumatological autoantibodies were associated with irAE organ specificity, severity, time or with patient survival.
Methods: This study utilized clinical data and plasma collected from 60 patients enrolled in a prospective phase II clinical trial of two doses of combination ICI (anti-CTLA4/anti-PD1) followed by anti-PD1 monotherapy for the treatment of advanced melanoma (PI Postow). IrAE severity was graded based on Common Terminology Criteria for Adverse Events (CTCAE) guidelines. Plasma was collected, frozen and stored at baseline and week six after treatment initiation, and later thawed and tested for anti-nuclear antibody (ANA), rheumatoid factor (RF) and anti-cyclic citrullinated peptide (CCP) using traditional methods. Characteristics were compared between seropositive and seronegative patients using Chi-square, t-tests and Wilcoxon rank-sum testing. The association of autoantibodies with timing of irAE was analyzed with Kaplan-Meier curves and log-rank testing, as was progression free and overall survival.
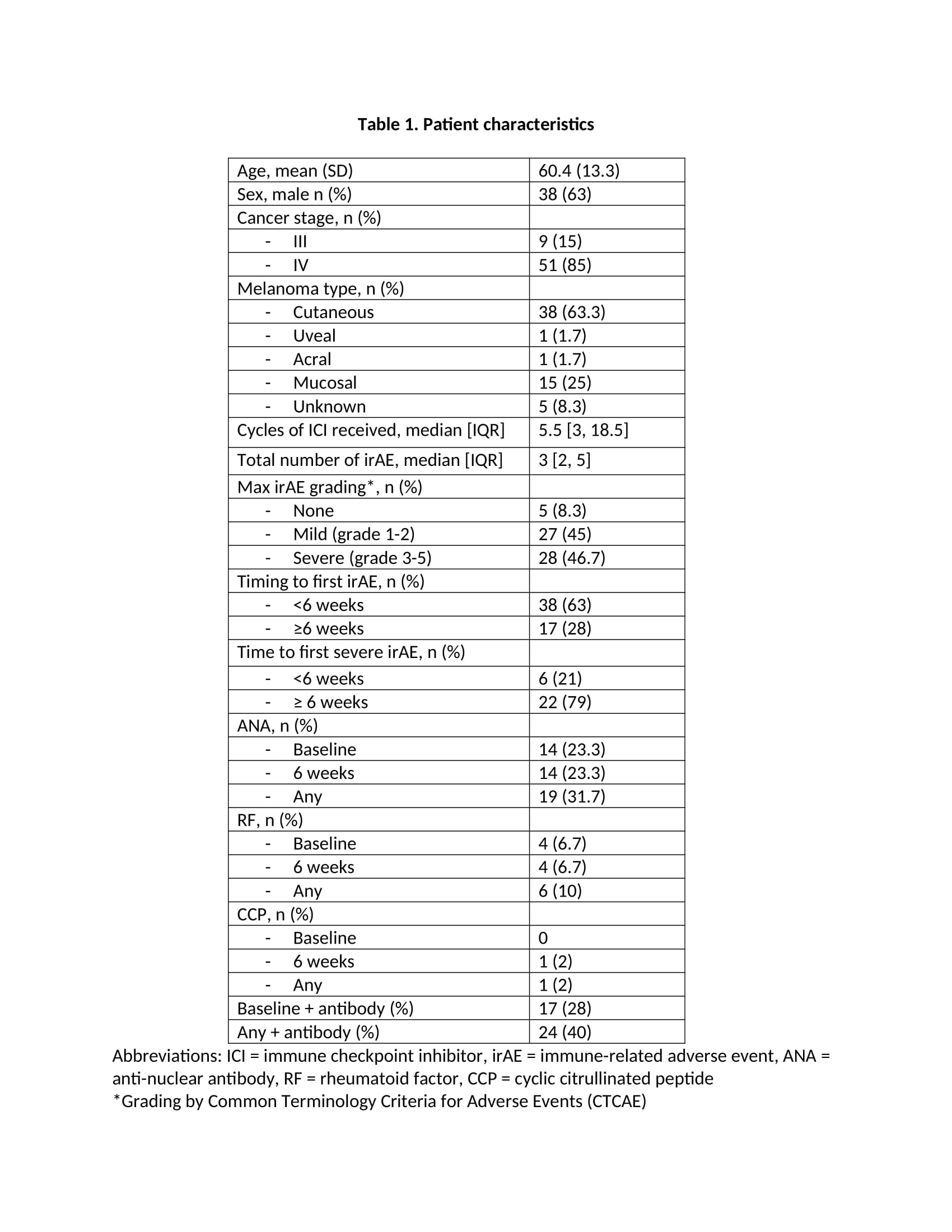
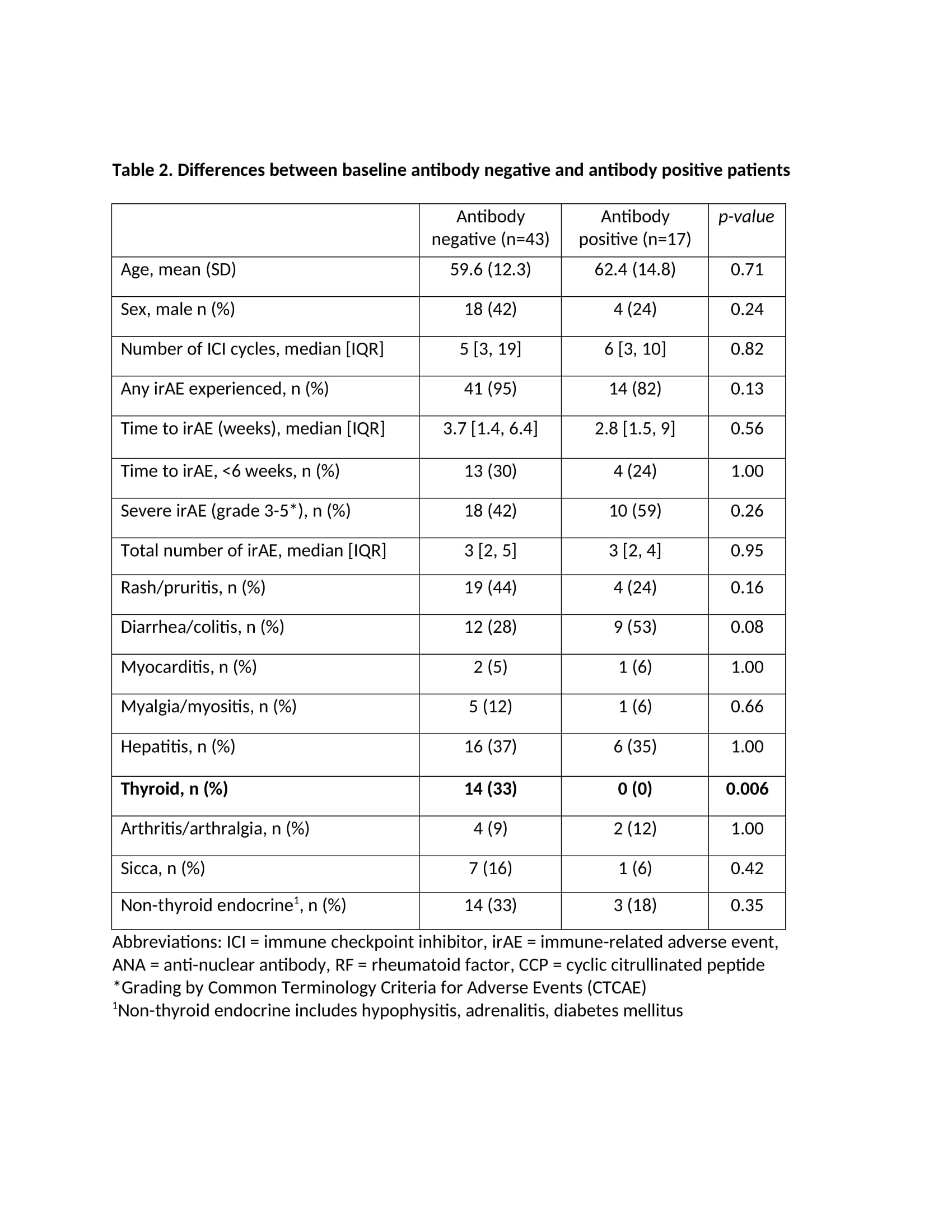
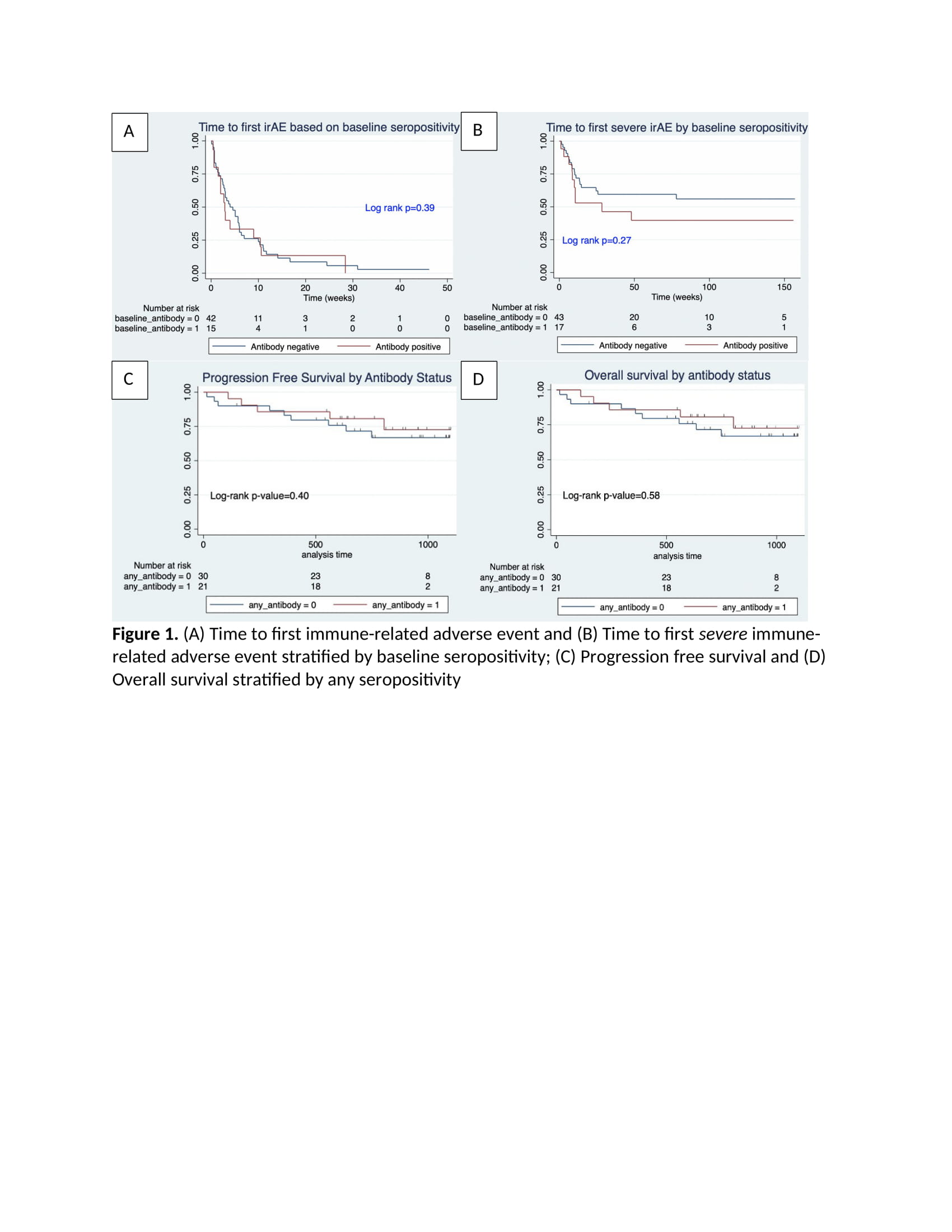
Results: Table 1 highlights the 60 patients included in the study with the mean age (SD) of 60.4 (SD 13.3), 63% male and 85% with stage IV melanoma. Ninety-two percent of patients experienced at least one irAE, with 45% experiencing grade 1-2, or mild irAE, and 46.7% experiencing grade 3-5, or severe irAE. Timing of the first irAE was less than six weeks in 69% of patients, most of which were dermatologic or gastrointestinal. Twenty-eight percent of patients had a baseline antibody (ANA, RF or CCP). There were no significant differences between the seropositive and seronegative patients in severity of irAE or type of irAE, except for thyroid irAE which was more common in the seronegative group (Table 2). There were no differences between the two groups in time to first irAE or first severe irAE (Figure 1), nor in progression free survival and overall survival.
Conclusion: Our study of melanoma patients did not find any association between ANA, RF and CCP positivity and irAE development, time to first irAE, irAE severity or survival. Seronegative patients were more likely to experience thyroid irAE than seropositive patients. These specific autoantibodies do not appear to be reliable biomarkers for irAE, although other autoantibodies or autoantibody profiles may prove to be more informative.
To cite this abstract in AMA style:
Ghosh N, Jannat-Khah D, Chan K, Postow M, Bass A. Do Specific Rheumatic Autoantibodies Predict Severity or Time to Onset of Immune-related Adverse Events from Checkpoint Inhibitors? [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/do-specific-rheumatic-autoantibodies-predict-severity-or-time-to-onset-of-immune-related-adverse-events-from-checkpoint-inhibitors/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/do-specific-rheumatic-autoantibodies-predict-severity-or-time-to-onset-of-immune-related-adverse-events-from-checkpoint-inhibitors/