Session Information
Date: Tuesday, November 19, 2024
Title: Abstracts: Muscle Biology, Myositis & Myopathies – Basic & Clinical Science II
Session Type: Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: The anti-Jo1 autoantibody (aJo1), targeting the histidyl-tRNA synthetase (HisRS) protein, is the most common diagnostic biomarker of the anti-synthetase syndrome (ASSD). So far, conflicting evidence supports the need of monitoring aJo1 levels over the disease course. The aim of this study was to analyze longitudinal levels of aJo1 in aJo1 positive patients with ASSD, and to explore their association with disease activity.
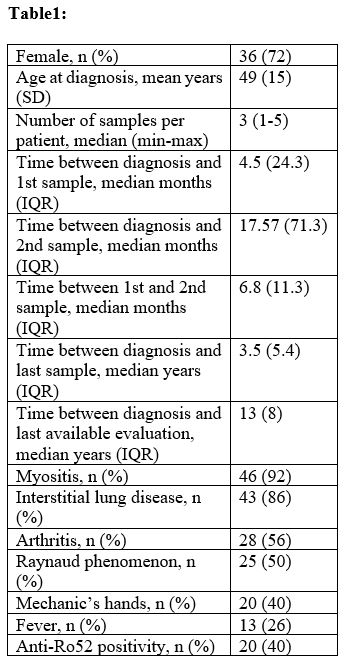
Methods: Serum samples (n=159) collected at time of diagnosis and during follow-up from aJo1 positive patients with ASSD (n=50) attending the Rheumatology clinic at Karolinska University Hospital, Stockholm, were analyzed by an in house ECLIA to measure both aJo1 and HisRS levels. Clinical and disease activity data (Table 1-2) was prospectively collected and retrospectively retrieved at each time of sampling and at last available evaluation. Remission-low disease activity was defined by physician global assessment (PhGA) ≤20 and/or physician’s judgement. Wilcoxon, Mann-Whitney, Spearman’s tests and mixed model regression for repeated measures were conducted.
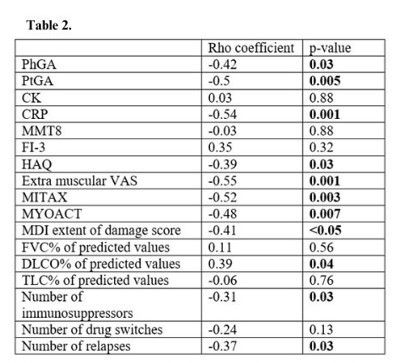
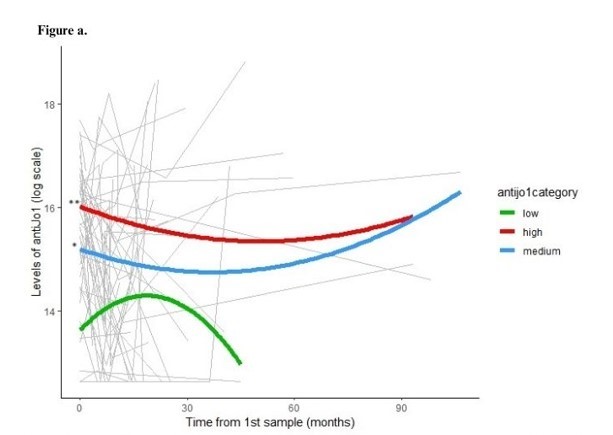
Results: Patients’ demographics and main characteristics are reported in Table 1. Three distinct groups of patients having high, medium and low aJo1 levels at first sampling were identified. Overall, the longitudinal levels of the three groups remained stable during the entire follow-up and rarely became undetectable (Fig.a). However, 17 patients were tested with undetectable aJo1 levels at least once in the whole observational period. Interestingly, HisRS levels were inversely correlated with aJo1 levels in the first sample (rho=-0.47, p=0.002) and over time (rho=-0.48; p< 0.001). When analyzing longitudinal disease activity, PhGA displayed a significant reduction during the entire follow up (p=0.03) and the levels of aJo1 predicted the change of PhGA over time (p< 0.001). Higher aJo1 levels in the first sample were found in patients with presence of anti-Ro52 autoantibodies (p=0.02) and interstitial lung disease (ILD) (p=0.005) and correlated to better outcomes at last available evaluation (Table 2). A statistically significant decrease of aJo1 levels was observed only between first and second sample (p=0.005). No correlation was found between delta aJo1 and delta outcome measures between these two samples. However, of 28 patients with available clinical data at time of first and second sample, those going into remission or low disease activity (n=22) during this time span (table 1) had higher levels of aJo1 in the first sample compared to those with moderate-high active disease (n=6) at time of second sample, and 86% of them remained in remission-low disease activity at the last evaluation.
Conclusion: In our study, longitudinal aJo1 levels were associated with PhGA over time. Furthermore, higher levels of aJo1 close to diagnosis correlated with occurrence of ILD and better outcome both at early and late-stage disease, suggesting that aJo1 may act as prognostic indicator in patients with aJo1 positive ASSD. Moreover, aJo1 levels displayed an inverse correlation with HisRS levels at each follow-up sample, indicating that they are bound in immune complexes. These preliminary findings need to be confirmed in larger cohorts.
To cite this abstract in AMA style:
Cavalli S, Espinosa-Ortega F, Adams R, Guy L, Preger C, Fernandes-Cerqueira C, Caporali R, Lundberg I, Notarnicola A. Do Levels of anti-Jo1 Autoantibodies Have a Prognostic Role? Longitudinal Assessment of anti-Jo1 and HisRS Protein Levels in a Cohort of anti-Jo1 Positive Patients with Anti-synthetase Syndrome [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/do-levels-of-anti-jo1-autoantibodies-have-a-prognostic-role-longitudinal-assessment-of-anti-jo1-and-hisrs-protein-levels-in-a-cohort-of-anti-jo1-positive-patients-with-anti-synthetase-syndrome/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/do-levels-of-anti-jo1-autoantibodies-have-a-prognostic-role-longitudinal-assessment-of-anti-jo1-and-hisrs-protein-levels-in-a-cohort-of-anti-jo1-positive-patients-with-anti-synthetase-syndrome/