Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Inflammatory-erosive arthritis is exacerbated by lymphatic dysfunction (1), and mast cells (MCs) regulate lymphatic vessel contractions via release of inflammatory and vasoactive mediators (2). In studies to directly assess this relationship, we identified a novel population of intravascular MCs embedded in the cellular architecture of murine joint-draining popliteal lymphatic vessels (PLVs) (3), along with perivascular MCs surrounding the PLV. Here, we investigated the phenotypic differences between PLV perivascular and intravascular MCs to elucidate their respective roles in regulating lymphatic function and inflammatory-erosive arthritis through genetic ablation and pharmacologic inhibition of MCs.
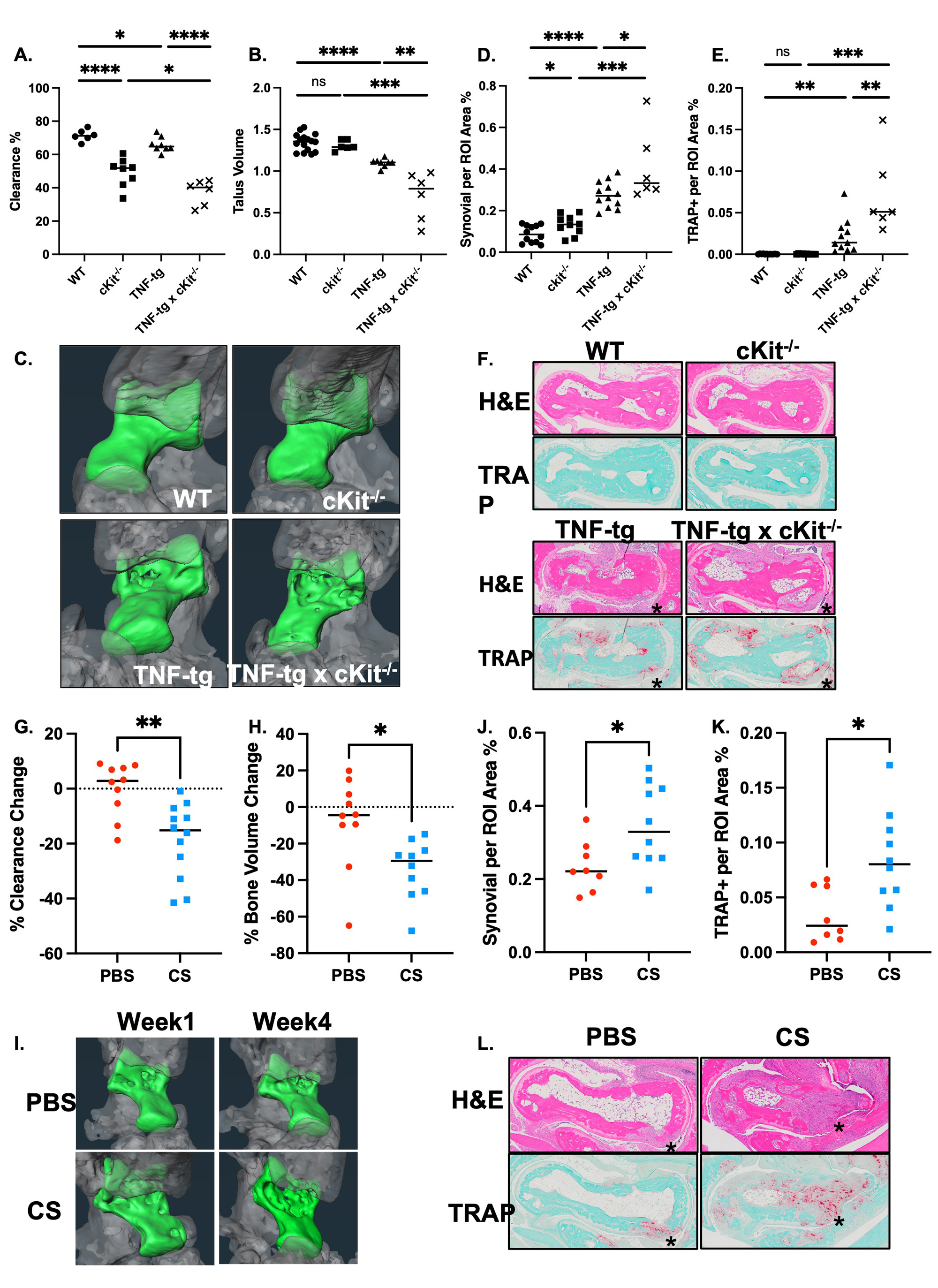
Methods: PLVs from WT, TNF-Tg, and KitW-sh/W-sh (cKit-/-) mice (4), which have a selective hematopoietic deficit in MCs, were harvested for whole mount immunofluorescent microscopy (WMIFM) and toluidine blue histochemistry to identify MCs. In silico analyses were performed on single-cell RNA sequencing (scRNAseq) datasets of PLV MCs (5) vs MC populations identified in the Mouse Cell Atlas (6). Near-infrared indocyanine green (NIR-ICG) imaging quantified lymphatic clearance function. Longitudinal micro-computed tomography (μCT) assessed bone erosions, while H&E and TRAP histology evaluated synovitis and osteoclasts of the afferent ankle. MC deficiency was studied in cKit-/- x TNF-Tg mice (n=3), while MC inhibition was investigated in TNF-Tg mice treated with cromolyn sodium (CS; 3.15mg/g/day/i.p., n=6) vssaline (n=5) for 3 weeks.
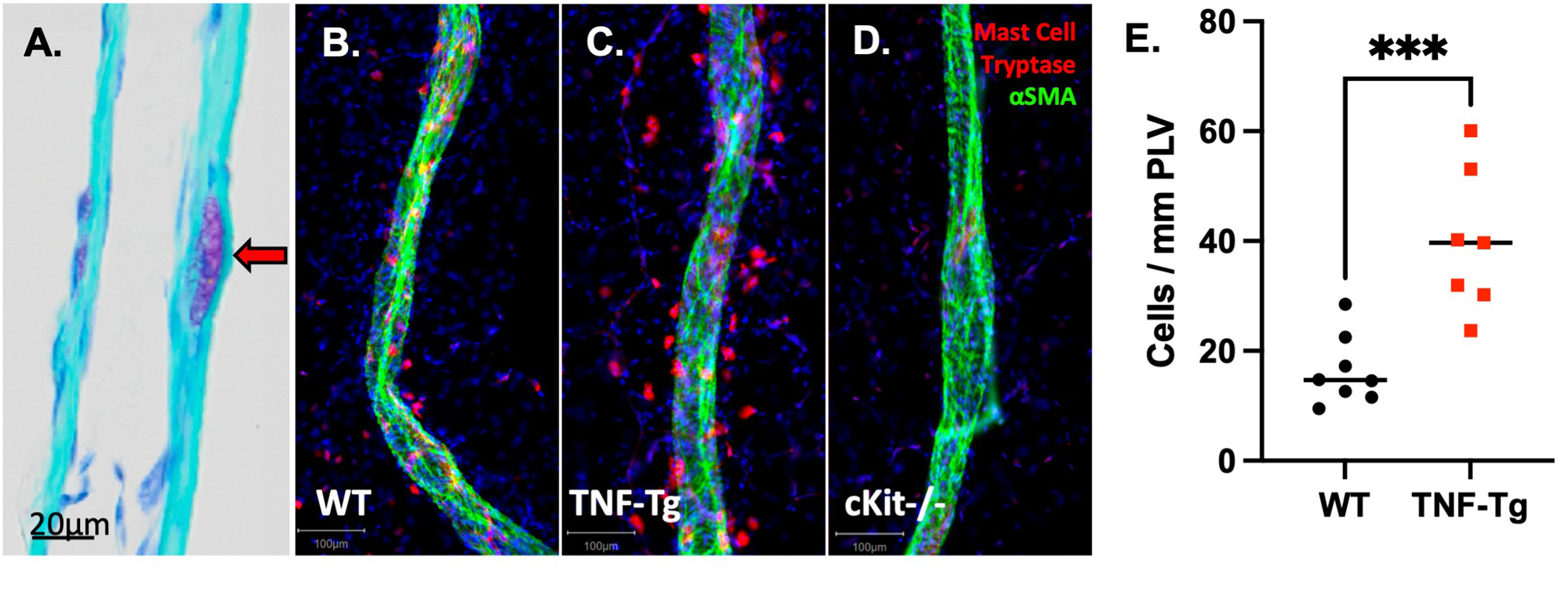
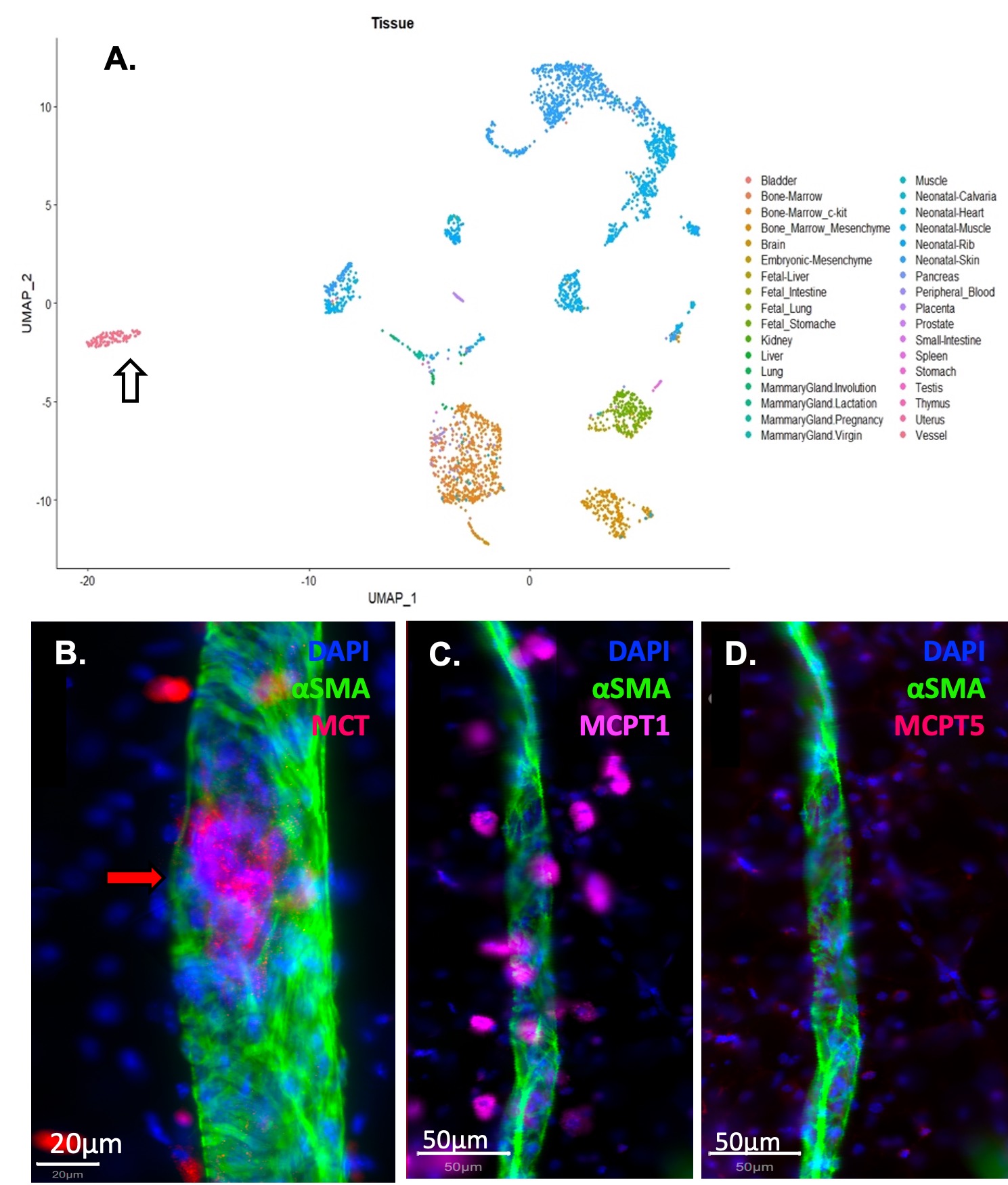
Results: WMIFM of MC-tryptase (MCT) confirmed increased numbers of perivascular MCs in TNF-Tg mice, and their complete absence in cKit-/-mice (Fig. 1). In silico scRNAseq analysis revealed a distinct population of PLV MCs vs known MCs in other mouse organs. WMIFM further demonstrated unique molecular signatures of perivascular (MCT+/MCTP1+/MCTP5–) vs intravascular (MCT+/MCTP1–/MCPT5–) MCs (Fig. 2). Remarkably, both CS treatment and cKit-/- x TNF-Tg mice exhibited decreased ICG clearance corresponding with increased bone erosions and synovitis vs their TNF-Tg controls (Fig. 3).
Conclusion: In this work, we characterized unique intravascular MCTP1– MCs in a cellular niche within the tissue structure [BK1] of murine joint-draining PLVs. These intravascular MCs are distinct from known MC subtypes, including perivascular MCTP1+ MCs, which are increased in TNF-Tg and absent in cKit-/- mice. Interestingly, both global MC deletion and inhibition decreased lymphatic function and exacerbated joint disease in TNF-Tg mice. These findings suggest subtype-specific MC regulation of lymphatic function through distinct cellular phenotypes, where MCTP1– intravascular MCs provide a protective role and selective deletion of MCTP1+ perivascular MCs restored lymphatic drainage and ameliorated inflammatory-erosive arthritis.
To cite this abstract in AMA style:
Peng Y, Kenney H, Bentley K, Xing L, Ritchlin C, Schwarz E. Distinct Perivascular and Intravascular Lymphatic Mast Cells and Their Role in Lymphatic Clearance, Joint Inflammation, and Bone Erosion in the TNF-Transgenic Murine Arthritis Model [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/distinct-perivascular-and-intravascular-lymphatic-mast-cells-and-their-role-in-lymphatic-clearance-joint-inflammation-and-bone-erosion-in-the-tnf-transgenic-murine-arthritis-model/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/distinct-perivascular-and-intravascular-lymphatic-mast-cells-and-their-role-in-lymphatic-clearance-joint-inflammation-and-bone-erosion-in-the-tnf-transgenic-murine-arthritis-model/