Session Information
Date: Sunday, November 8, 2020
Title: Pediatric Rheumatology – Clinical I: Treatment of JIA (1492–1496)
Session Type: Abstract Session
Session Time: 4:00PM-4:50PM
Background/Purpose: Treatment response in JIA is often viewed as a binary outcome: response or non-response, usually assessed using composite, multidimensional measures, such as the juvenile arthritis disease activity score (JADAS). Within a composite outcome, there are likely different, identifiable patterns of response that cluster within subgroups of children and young people (CYP). Identifying these subgroups could assist the tailoring of stratified treatment approaches in JIA and offer further insights into understanding treatment response.
This study aimed to identify subgroups of CYP defined by different trajectories of individual JADAS components in the first year following initiation of methotrexate (MTX).
Methods: The discovery cohort for subgroup identification included MTX-naïve CYP with JIA at the point of MTX initiation, enrolled prior to January 2018 to either the BSPAR Etanercept Cohort Study (BSPAR-ETN) or the Biologics for Children with Rheumatic Diseases Study (BCRD), nationwide pharmacological registers. The Childhood Arthritis Prospective Study (CAPS), a multicentre JIA inception cohort, was used for verification. JADAS components (active joint count, physician’s global assessment (PGA, 0-10cm), parental global evaluation (PGE, 0-10cm) and standardised ESR (0-10) were calculated based on data collected in the year following MTX initiation and log1p transformed for analysis.
Multivariate group-based trajectory models were used to explore MTX response clusters. Optimal models were selected based on a combination of model fit (BIC, relative entropy, average posterior probabilities), parsimony and clinical plausibility. Model development and selection were repeated independently in the verification cohort.
Results: The discovery cohort included 658 CYP, and in the verification cohort n=537. The majority were female (68%, 70%) and of white ethnicity (86%, 82%), with RF-negative JIA (35%, 33%), respectively.
Six disease trajectories following MTX were identified in the discovery cohort: Two groups improved across all JADAS components: Fast improvers (11%) and Slow Improvers (16%). A large group maintained persistent disease overall (44%). Two groups maintained one persistent disease feature despite otherwise improvement: Persistent PGA (8%) and Persistent PGE (13%). A final group experienced disease relapse (7%) (Figure 1). These results were verified in the CAPS cohort, with similar subgroups and group proportions identified, except for the relapse group, which was not evident in the CAPS cohort.
Conclusion: We identify six clusters of CYP following initiation of MTX, each with differing patterns of disease activity, suggesting a simple responder/non-responder analysis at a set point may be over-simplistic. Common patterns were identified across multiple, large, UK cohorts. Understanding both clinical factors associated with, and biological mechanisms underpinning, these subgroups would aid stratified treatment decisions in JIA.
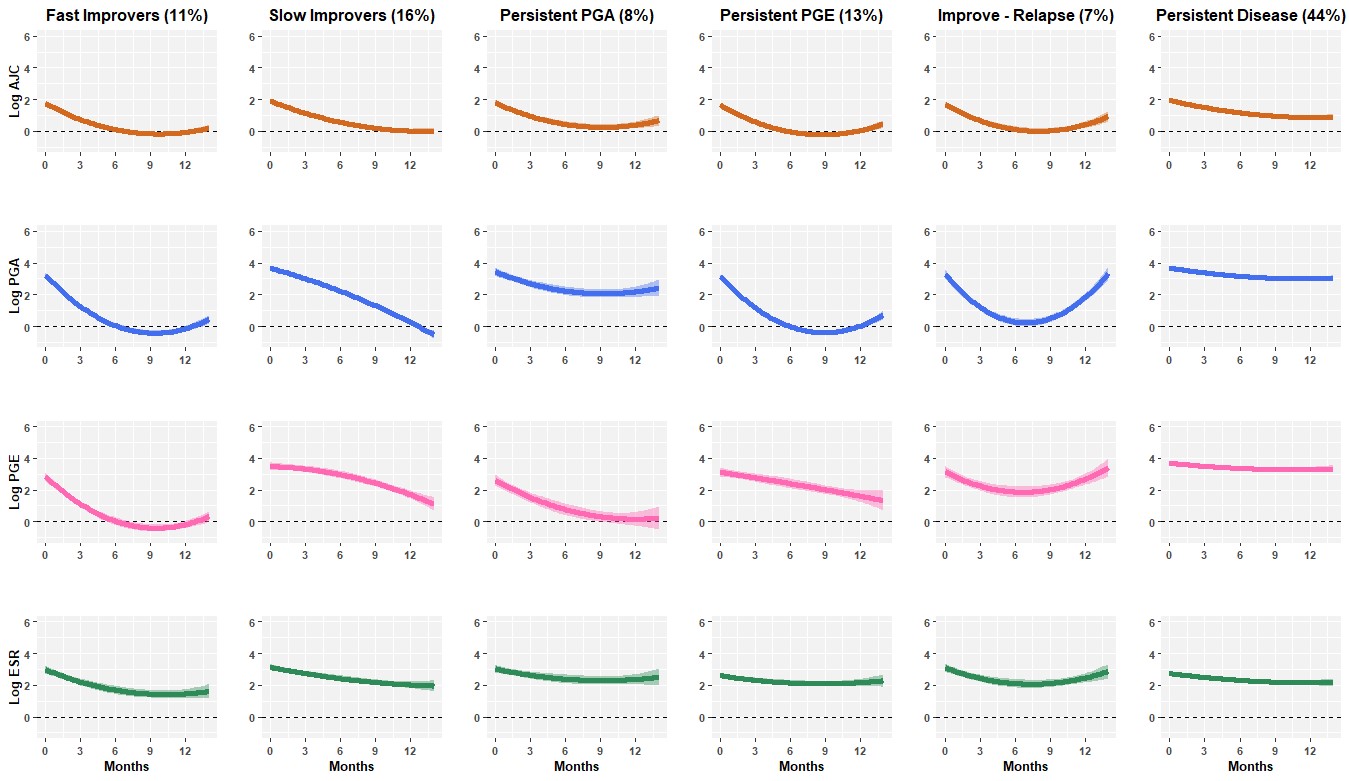 Figure 1. Clusters of MTX response identified in children and young people with JIA enrolled to the BSPAR-ETN or BCRD studies
Figure 1. Clusters of MTX response identified in children and young people with JIA enrolled to the BSPAR-ETN or BCRD studies
To cite this abstract in AMA style:
Shoop-Worrall S, Hyrich K, Wedderburn L, Thomson W, Geifman N. Distinct Patient-level Patterns of Response to Methotrexate in Juvenile Idiopathic Arthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/distinct-patient-level-patterns-of-response-to-methotrexate-in-juvenile-idiopathic-arthritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/distinct-patient-level-patterns-of-response-to-methotrexate-in-juvenile-idiopathic-arthritis/
