Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Japanese guidelines for managing gout and hyperuricemia recommend the initiation of urate-lowering therapy (ULT) to prevent gouty arthritis in subjects having asymptomatic hyperuricemia with serum uric acid (sUA) ≥8.0 mg/dL under certain conditions [Yamanaka H. Japan Med Assoc J. 2012;55:324-9.]. This is a distinctive difference from policies in Europe and the US. However, we lack clear documentation of the extent of clinical treatment for asymptomatic hyperuricemic subjects and of correlations between such treatment and the prevention of gouty arthritis. This study evaluated the association between disease control, defined as the relationship among prescription of ULT, occurrence of gouty arthritis, and level of sUA after 1 year, and gouty arthritis in subjects with asymptomatic hyperuricemia newly detected at medical check-up.
Methods: This research is based on Japanese health insurance claims and medical check-up data from April 2012 through June 2019. We identified subjects who had sUA ≥8.0 mg/dL in one or more medical check-ups from April 1, 2013 to March 31, 2016. The earliest available measurement date during that period was considered the index date. Subjects of analysis were health insurance subscribers whose records did not show the disease name of “gout” or “asymptomatic hyperuricemia” and for whom ULT had not been prescribed during the 1-year period prior to the index date. From the index date to the medical check-up 1 year later (follow-up date) was considered Period 1, and from the day after the follow-up date was considered Period 2. Disease control was investigated during Period 1, and disease burden (incidence rate of gouty arthritis) was assessed in relation to disease control during Period 2 (Figure 1).
Results: The analysis population consisted of 19,261 health insurance subscribers who met the eligibility criteria. During Period 1, ULT was initiated in 8.4% of that group (1,625 /19,261). Gouty arthritis was experienced by 3.1% (597/19,261), of whom 32.3% (193/597) were not under treatment with ULT. For 90.6% (17,443/19,261), no ULT was prescribed and no gouty arthritis was experienced during Period 1. Within that group, 40.4% (7,049/17,443) showed sUA ≥8.0 mg/dL on the follow-up date. Only 2.3% (438/19,261) had been prescribed ULT and had achieved sUA ≤6.0 mg/dL by the follow-up date (Figure 2). During Period 2, the incidence rate of gouty arthritis was calculated for each type of disease control assessed during Period 1. Both for asymptomatic hyperuricemia and for gout in subjects who had experienced at least one incident of gouty arthritis during Period 1, the incidence rate of gouty arthritis during Period 2 was lower in subjects who had sUA ≤6.0 mg/dL than in those whose sUA was >6.0 mg/dL, with or without ULT (Figure 3).
Conclusion: Of those subscribers with newly detected hyperuricemia according to medical check-up records, only 2.3% had been prescribed ULT and had achieved sUA ≤6.0 mg/dL for the 1-year period. Both for asymptomatic hyperuricemia and for gout, the incidence rate of gouty arthritis was lower in subjects whose sUA was ≤6.0 mg/dL than those whose sUA was >6.0 mg/dL, with or without ULT. Further analysis by adjusting for confounding will be required to discuss causality.
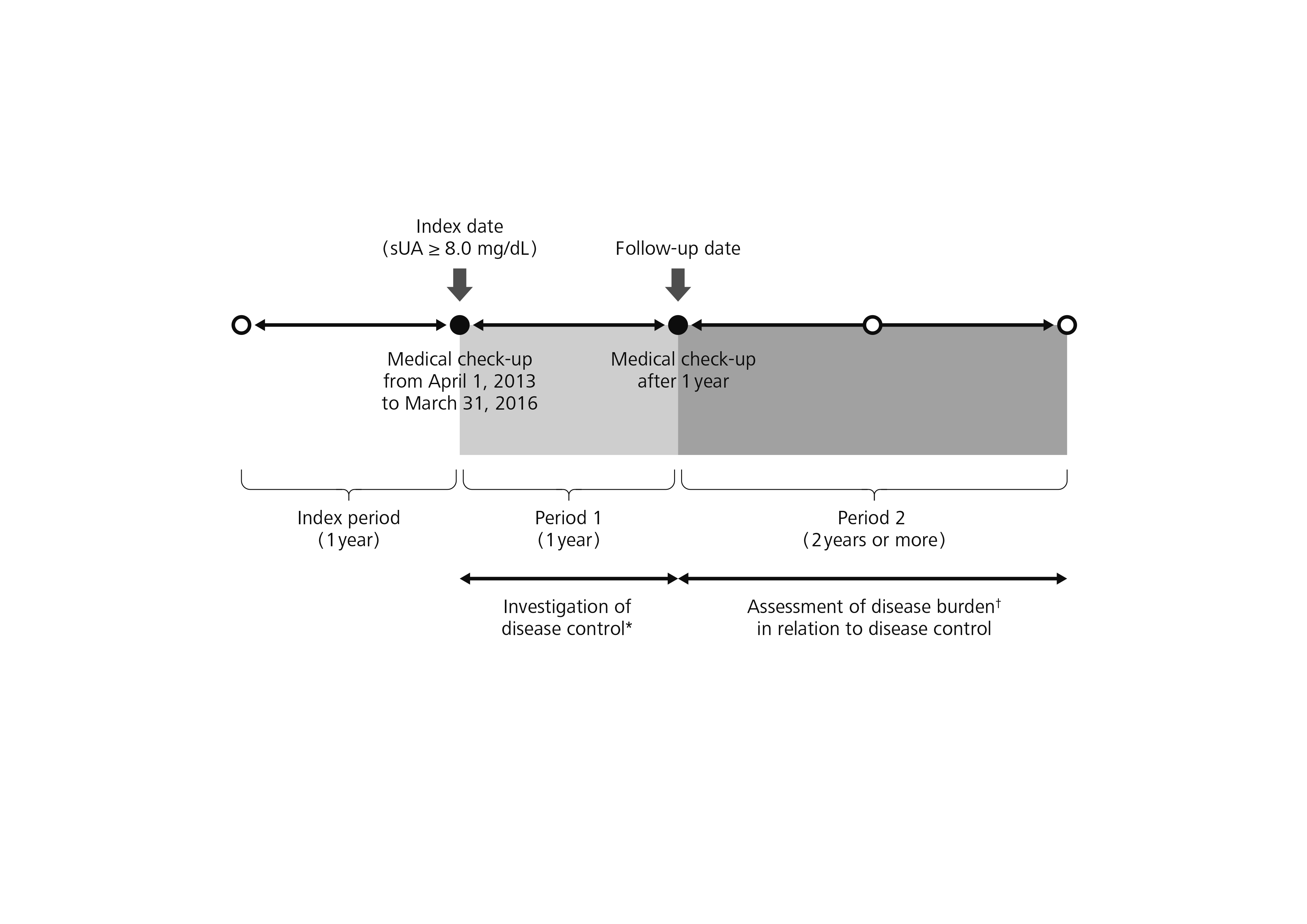 Figure 1 Study design * Disease control: Relationships among prescription of ULT, occurrence of gouty arthritis, and level of sUA after 1 year † Incidence rate of gouty arthritis sUA, serum uric acid; ULT, urate-lowering therapy
Figure 1 Study design * Disease control: Relationships among prescription of ULT, occurrence of gouty arthritis, and level of sUA after 1 year † Incidence rate of gouty arthritis sUA, serum uric acid; ULT, urate-lowering therapy
 Figure 2 Event tree analysis of disease control from index date to follow-up date (Period 1) Parentheses indicate the number of subjects. For percentages, the denominator was 19,261 subscribers. sUA, serum uric acid; ULT, urate-lowering therapy
Figure 2 Event tree analysis of disease control from index date to follow-up date (Period 1) Parentheses indicate the number of subjects. For percentages, the denominator was 19,261 subscribers. sUA, serum uric acid; ULT, urate-lowering therapy
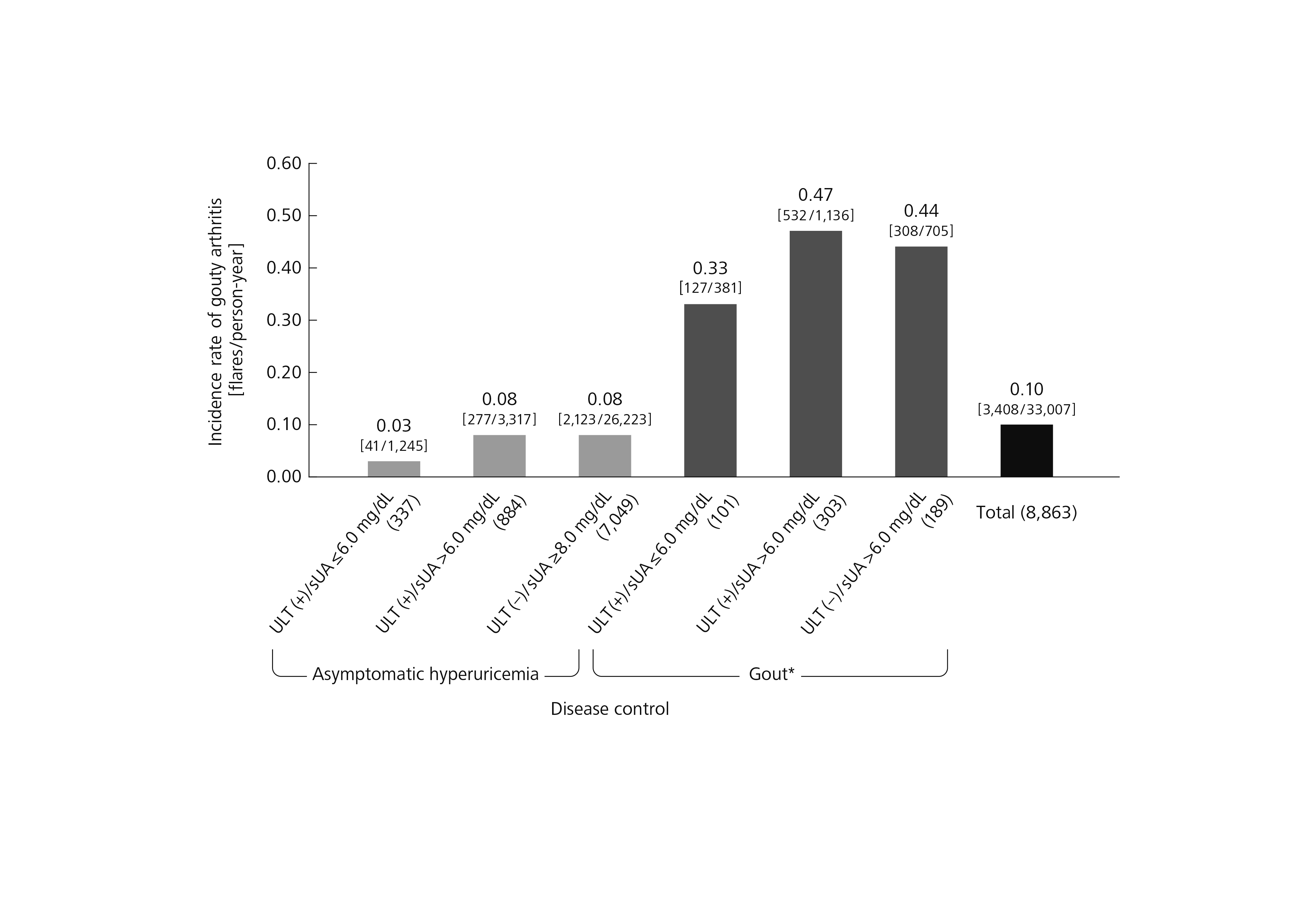 Figure 3 Incidence rate of gouty arthritis during Period 2 Parentheses indicate the number of subjects. Brackets indicate the number of flares/person-years. * Subjects who had experienced at least one incident of gouty arthritis during Period 1. sUA, serum uric acid; ULT, urate-lowering therapy
Figure 3 Incidence rate of gouty arthritis during Period 2 Parentheses indicate the number of subjects. Brackets indicate the number of flares/person-years. * Subjects who had experienced at least one incident of gouty arthritis during Period 1. sUA, serum uric acid; ULT, urate-lowering therapy
To cite this abstract in AMA style:
Koto R, Nakajima A, Horiuchi H, Yamanaka H. Disease Control of Hyperuricemia Newly Detected by Medical Check-up: A Retrospective Cohort Study of Health Insurance Claims Data in Japan [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/disease-control-of-hyperuricemia-newly-detected-by-medical-check-up-a-retrospective-cohort-study-of-health-insurance-claims-data-in-japan/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/disease-control-of-hyperuricemia-newly-detected-by-medical-check-up-a-retrospective-cohort-study-of-health-insurance-claims-data-in-japan/
