Session Information
Date: Sunday, November 12, 2023
Title: (0380–0422) RA – Diagnosis, Manifestations, and Outcomes Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Despite major advances in RA treatment, a substantial number of patients (estimated at 6-21%1) are refractory to multiple advanced therapies. Well-defined refractory RA (reRA) criteria and thoughtful selection of controls are essential to ensure that observed differences are truly due to refractory disease and not to confounding variables. In this study, we aimed to identify factors associated with reRA and characterize differences in disease burden/patient experiences between reRA and matched nonrefractory controls, from over 20 years of real-world data.
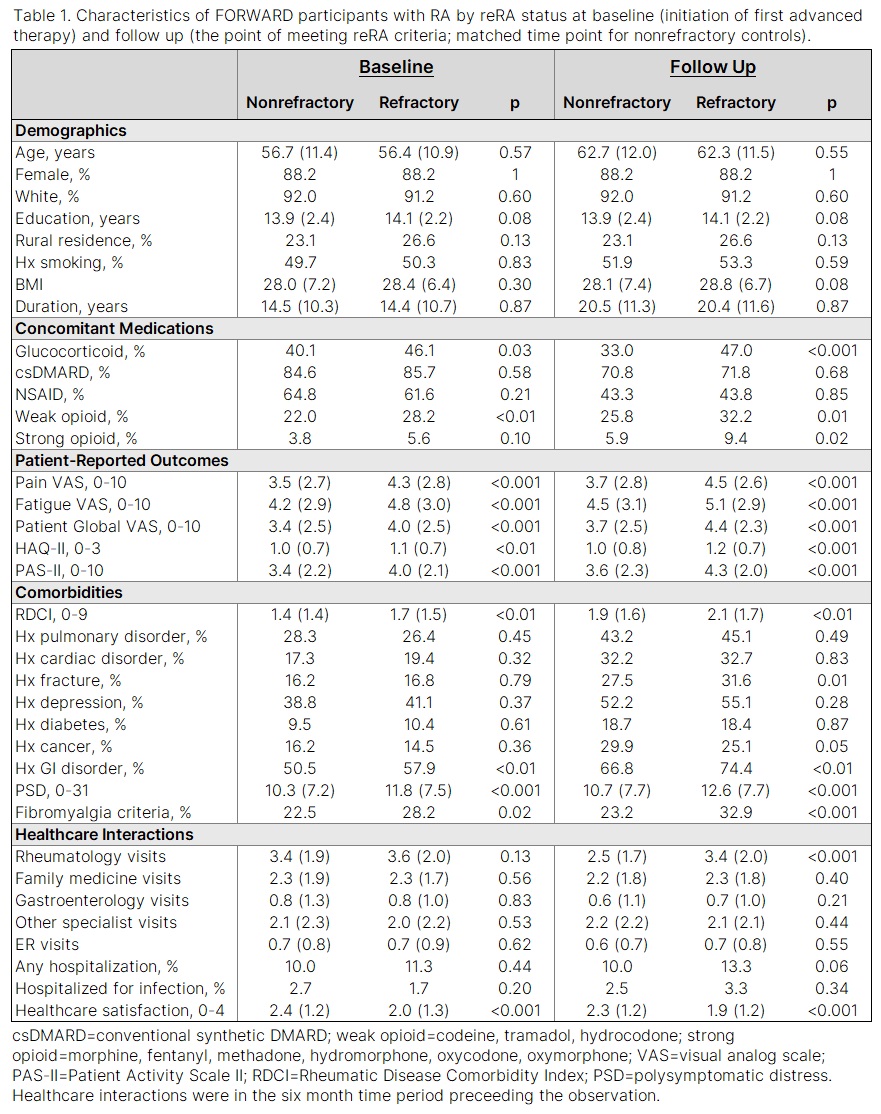
Methods: Data were provided by adults with RA in the FORWARD Databank from 1998 to 2019. Participants with no history of biologic (bDMARD) or targeted synthetic DMARD (tsDMARD) use at study entry but with subsequent exposure to one or more of these advanced therapies were included. The reRA group included participants with exposure to ≥3 advanced therapies during observation, with ≥1 TNF inhibitor (TNFi) and ≥1 tsDMARD or non-TNFi bDMARD. The nonrefractory group included participants with continued use of their first advanced therapy for at least two years, and who never exceeded two advanced therapies during observation. Refractory and nonrefractory participants were matched 1:1 on age, sex, RA duration, calendar year, and observation time. Descriptive statistics were calculated at baseline (initiation of first advanced therapy) and at follow up (the point of meeting reRA criteria; matched time point for nonrefractory controls). Significance was assessed with Chi-square and t-tests, as appropriate. Covariates with p< 0.1 were included in multivariable logistic regression models for each time point.
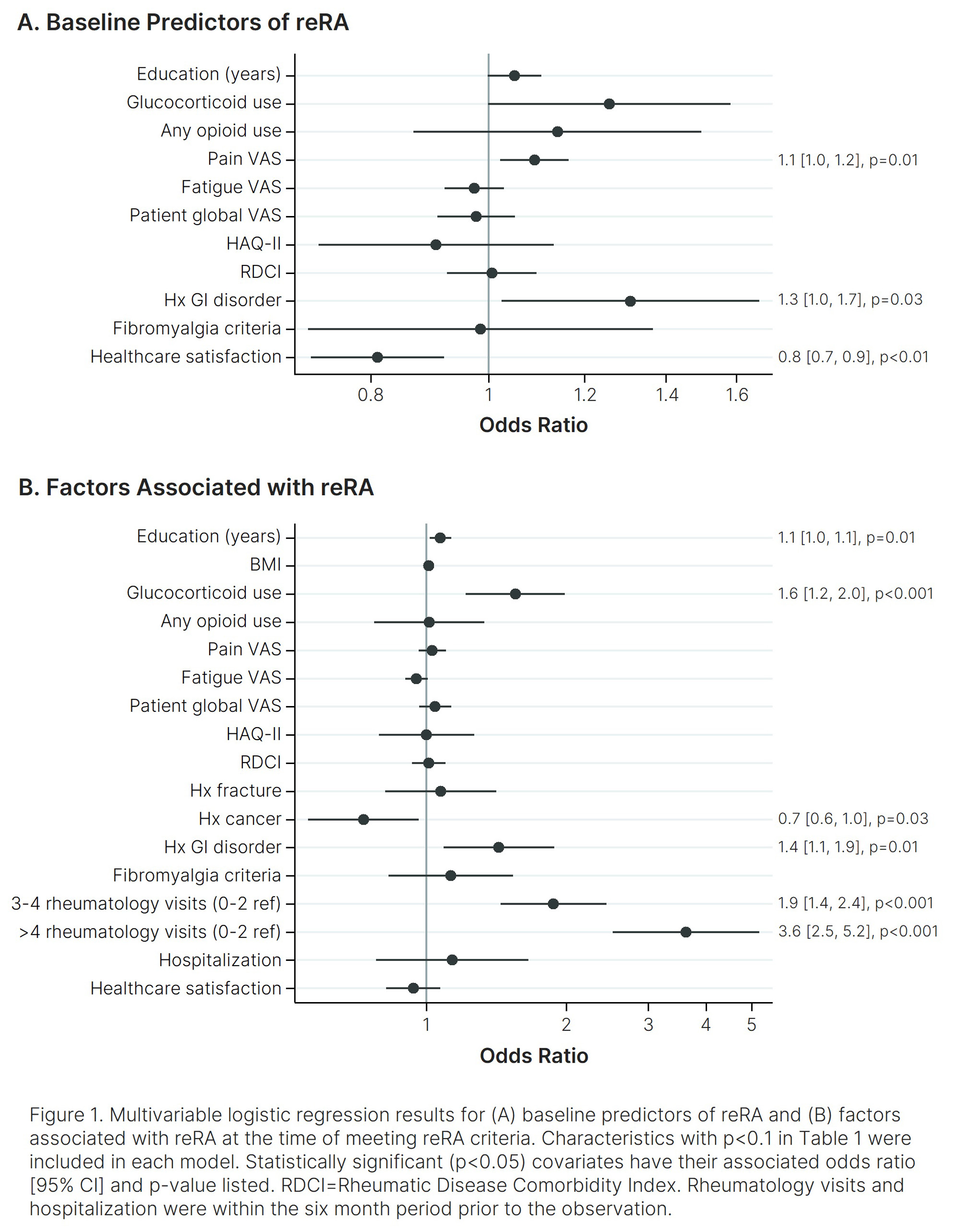
Results: Of 6,575 participants who met study inclusion criteria, 718 (10.9%) met reRA criteria. Of those, 692 were matched 1:1 to nonrefractory controls, for a total of 1,384 participants included in the study. In univariate analyses (Table 1), all patient-reported outcomes (PROs) were significantly worse among the reRA group at both time points. Glucocorticoid use, opioid use, Rheumatic Disease Comorbidity Index (RDCI), gastrointestinal (GI) disorder, and polysymptomatic distress (PSD) were also all higher for the reRA group at both time points, and healthcare satisfaction was lower. In multivariable analyses (Figure 1) higher pain, GI disorder, and lower healthcare satisfaction were all associated with future incidence of reRA. Higher education, corticosteroid use, GI disorder, and more rheumatology visits were associated with reRA. History of cancer was associated with nonrefractory status.
Conclusion: These results demonstrate that reRA is associated with significant disease burden and unmet healthcare needs, as evidenced by lower healthcare satisfaction, higher rates of glucocorticoid and opioid use, greater comorbidity and symptom burdens, and more rheumatology visits. These findings underscore the importance of well-defined reRA criteria and the need for further investigation into this unique RA phenotype to identify targeted treatment strategies and ultimately improve outcomes.
1. Melville, A. R. et al. Drugs80, 849–857 (2020)
To cite this abstract in AMA style:
Wipfler K, Han B, Pedro S, Sbarigia U, Zazzetti F, Sheahan A, Lin I, Katz P, Alemao E, Michaud K. Disease Burden, Patient Experiences, and Unmet Needs in Refractory Rheumatoid Arthritis: Insights from 20 Years of Real-World Data [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/disease-burden-patient-experiences-and-unmet-needs-in-refractory-rheumatoid-arthritis-insights-from-20-years-of-real-world-data/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/disease-burden-patient-experiences-and-unmet-needs-in-refractory-rheumatoid-arthritis-insights-from-20-years-of-real-world-data/