Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Timely diagnosis of children and young adults with rheumatologic disorders remains a global challenge especially in lower-resource settings. There is limited access to diagnostic laboratory tools such as antinuclear antibody (ANA) testing which is necessary to confirm a diagnosis of several rheumatic disorders. Easier availability of ANA testing will allow for timely initiation of life-saving treatment and improve outcomes. Thus, our objective was to develop a lower cost, accurate and scalable dried blood spot (DBS) ANA testing option that can be effectively used in lower-resource settings in Africa and other parts of the world.
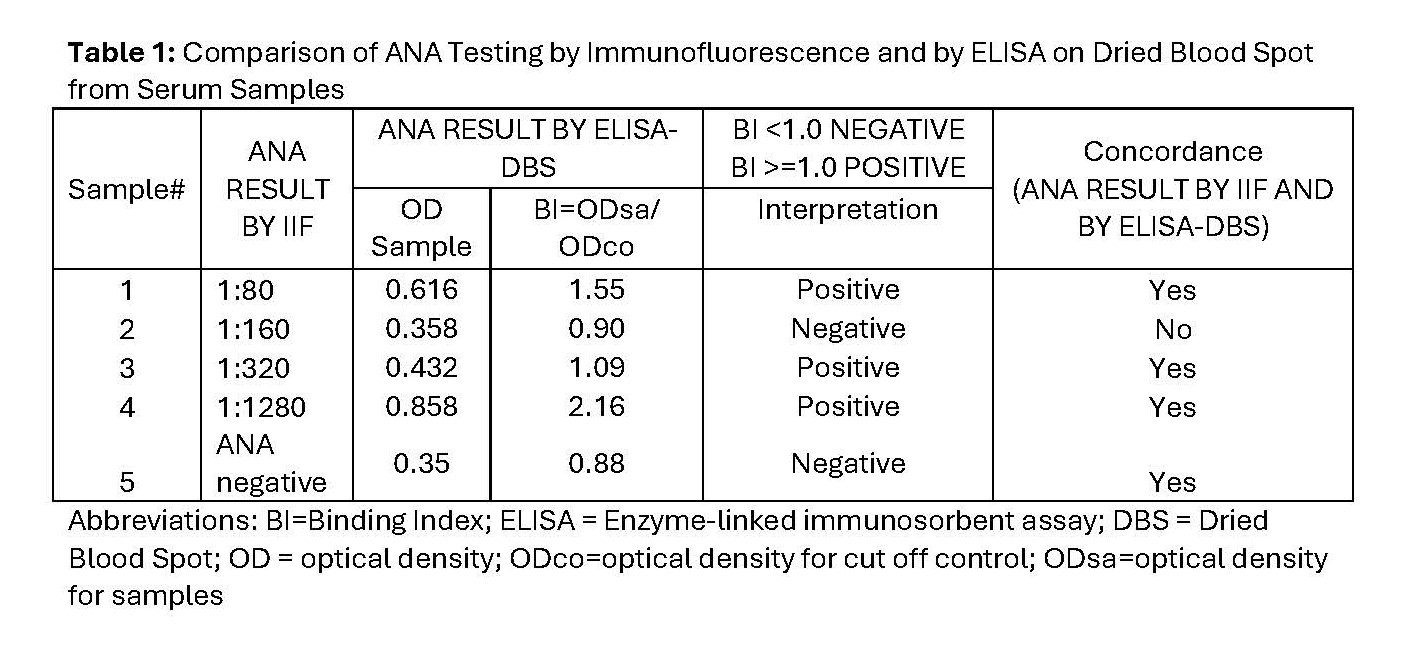
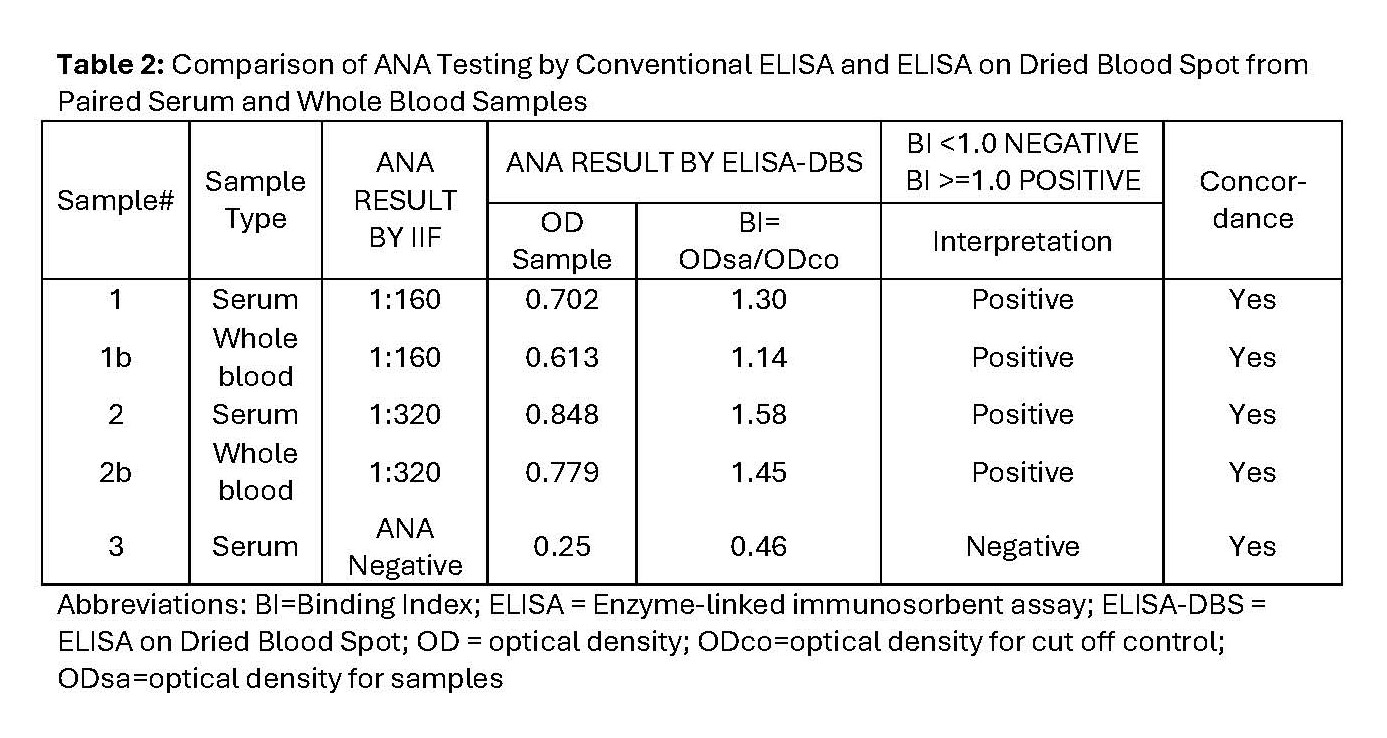
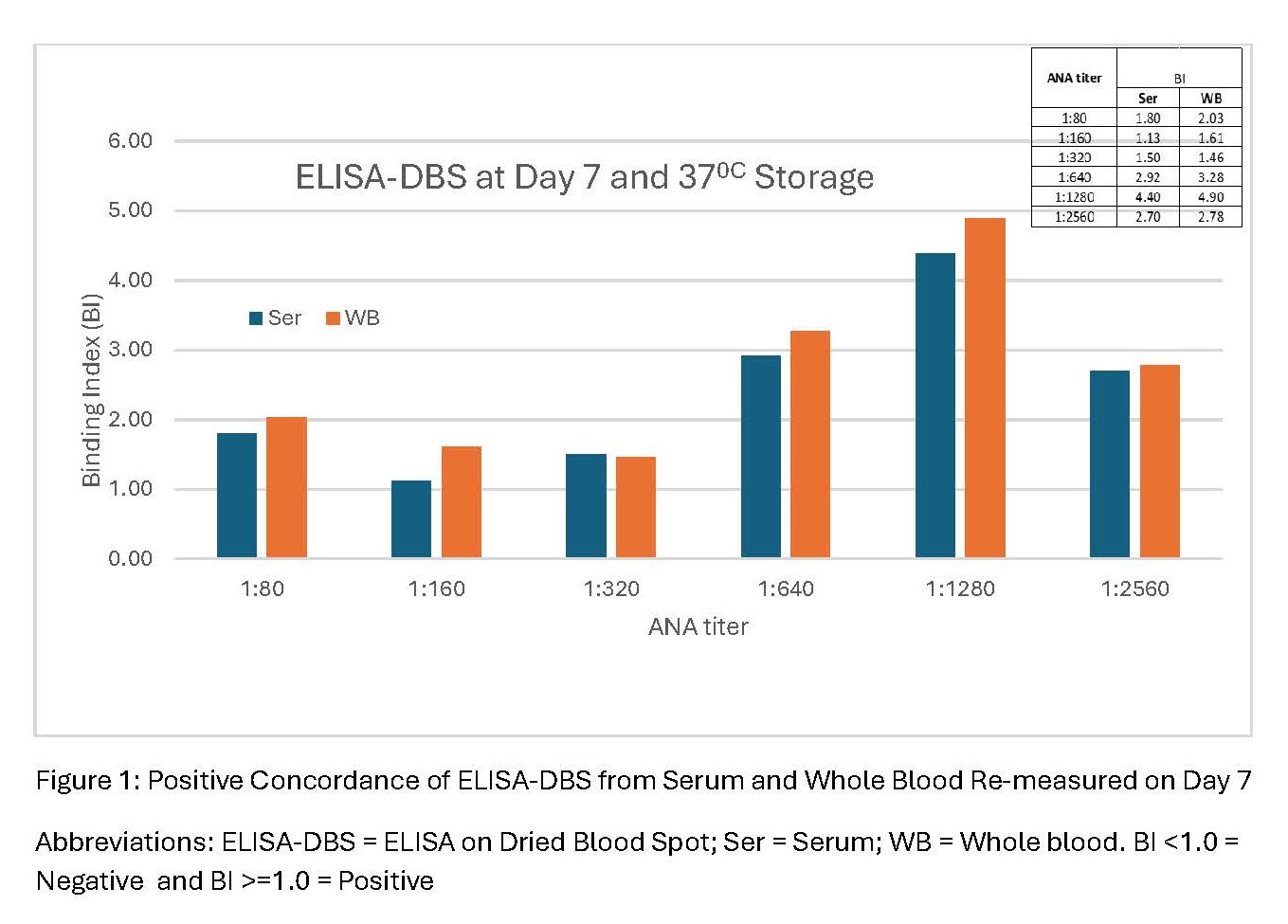
Methods: De-identified cross-sectional blood samples collected via venipuncture were tested for the presence of ANA by the gold standard immunofluorescence (IIF) and ELISA on DBS (ELISA-DBS). For ELISA-DBS, serum or whole blood (WB) samples were transferred to a protein-optimized DBS paper matrix and eluted overnight prior to ELISA testing (measured as positive or negative). Initially, the presence of ANA in serum samples was tested concurrently by IIF (1:80 – 1:1280 titers) and ELISA-DBS. Following this, paired serum and WB samples of varying ANA titers by IIF (1:80 – 1:2560) were also tested by ELISA-DBS. Then, ANA results by IIF and by ELISA-DBS tests were compared for varying (10 – 75 microliter) and stable (30 microliter) DBS sample quantities for volume and stability studies respectively. Comparison of ELISA-DBS from paired serum and WB samples was also performed following exposure of DBS samples to room temperature and 370C, and on prolonged DBS sample storage (24hours/7days/14days) prior to ELISA-DBS testing.
Results: Comparison of serum samples concurrently tested for ANA by IIF and by ELISA-DBS showed 80% concordance (N = 4, Table 1). There was 100% concordance of paired serum and WB samples of varying ANA titers by IIF with ELISA-DBS (N= 3, Table 2). ELISA-DBS from paired 30 microliter serum and WB samples at 24hrs/7days/14days yielded 83/100/67% concordance in ANA results respectively (Figure 1).
Conclusion: Our pilot study demonstrates that ANA testing from DBS is feasible with good concordance at varying ANA titers and with temperature excursions. Our novel ANA testing by ELISA-DBS could aid earlier diagnoses of rheumatic diseases in lower resource settings.
To cite this abstract in AMA style:
Ogbu E, Florea S, Diorio D, Roy S, Sharma D, Henrickson M, Vega-Fernandez P, Migowa A, Ranganathan S, brunner h. Development of a Novel Dried Blood Spot Method to Improve Capacity for ANA Testing in Lower-Resource Settings [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/development-of-a-novel-dried-blood-spot-method-to-improve-capacity-for-ana-testing-in-lower-resource-settings/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-a-novel-dried-blood-spot-method-to-improve-capacity-for-ana-testing-in-lower-resource-settings/